Circulating Extracellular Vesicles and Endothelial Damage in Sickle Cell Disease
- PMID: 33013455
- PMCID: PMC7495019
- DOI: 10.3389/fphys.2020.01063
Circulating Extracellular Vesicles and Endothelial Damage in Sickle Cell Disease
Abstract
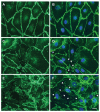
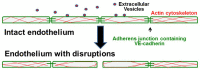
Endothelial damage is central to the pathogenesis of many of the complications of sickle cell disease. Circulating extracellular vesicles (EVs) have been implicated in modulating endothelial behavior in a variety of different, diseases with vascular pathologies. As seen in other hemolytic diseases, the plasma of sickle cell patients contains EVs of different sizes and cellular sources. The medium-sized vesicles (microparticles) primarily derive from mature red blood cells and platelets; some of these EVs have procoagulant properties, while others stimulate inflammation or endothelial adhesiveness. Most of the small EVs (including exosomes) derive from erythrocytes and erythrocyte precursors, but some also originate from platelets, white blood cells, and endothelial cells. These small EVs may alter the behavior of target cells by delivering cargo including proteins and nucleic acids. Studies in model systems implicate small EVs in promoting vaso-occlusion and disruption of endothelial integrity. Thus, both medium and small EVs may contribute to the increased endothelial damage in sickle cell disease. Development of a detailed understanding of the composition and roles of circulating EVs represents a promising approach toward novel predictive diagnostics and therapeutic approaches in sickle cell disease.
Keywords: endothelial damage; exosomes; extracellular vesicle; microvesicle; sickle cell disease.
Copyright © 2020 Lapping-Carr, Gemel, Mao and Beyer.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

