Role of Renin-Angiotensin System Components in Atherosclerosis: Focus on Ang-II, ACE2, and Ang-1-7
- PMID: 33013457
- PMCID: PMC7494970
- DOI: 10.3389/fphys.2020.01067
Role of Renin-Angiotensin System Components in Atherosclerosis: Focus on Ang-II, ACE2, and Ang-1-7
Abstract
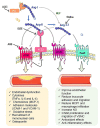
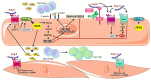
Atherosclerosis is the leading cause of vascular disease worldwide and contributes significantly to deaths from cardiovascular complications. There is a remarkably close relationship between atherosclerotic plaque formation and the activation of renin-angiotensin system (RAS). However, depending on which RAS pathway is activated, pro- or anti-atherogenic outcomes may be observed. This brief review focuses on the role of three of the most important pieces of RAS axis, angiotensin II (Ang-II), angiotensin converting enzyme type 2 (ACE2), and angiotensin 1-7 (Ang-1-7) and their involvement in atherosclerosis. We focused on the effects of these molecules on vascular function and inflammation, which are important determinants of atherogenesis. Furthermore, we highlighted potential pharmacological approaches to treat this disorder.
Keywords: angiotensin 1–7; angiotensin II; angiotensin converting enzyme type 2; atherosclerosis; endothelial dysfunction; inflammation.
Copyright © 2020 Silva, França-Falcão, Calzerra, Luz, Gadelha, Balarini and Queiroz.
Figures
References
-
- Anguiano L., Riera M., Pascual J., Valdivielso J. M., Barrios C., Betriu A., et al. (2016). Circulating angiotensin converting enzyme 2 activity as a biomarker of silent atherosclerosis in patients with chronic kidney disease. Atherosclerosis 253, 135–143. 10.1016/j.atherosclerosis.2016.08.032, PMID: - DOI - PubMed
-
- Canault M., Leroyer A. S., Peiretti F., Lesèche G., Tedgui A., Bonardo B., et al. (2007). Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-alpha converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am. J. Pathol. 171, 1713–1723. 10.2353/ajpath.2007.070021, PMID: - DOI - PMC - PubMed
-
- Canault M., Peiretti F., Kopp F., Bonardo B., Bonzi M. F., Coudeyreet J. C., et al. (2006). The TNF alpha convert-ing enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis 187, 182–191. 10.1016/j.atherosclerosis.2005.08.031, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

