Targeting Ca2 + Handling Proteins for the Treatment of Heart Failure and Arrhythmias
- PMID: 33013458
- PMCID: PMC7498719
- DOI: 10.3389/fphys.2020.01068
Targeting Ca2 + Handling Proteins for the Treatment of Heart Failure and Arrhythmias
Abstract
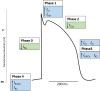
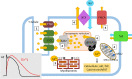
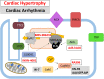
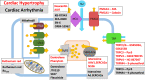
Diseases of the heart, such as heart failure and cardiac arrhythmias, are a growing socio-economic burden. Calcium (Ca2+) dysregulation is key hallmark of the failing myocardium and has long been touted as a potential therapeutic target in the treatment of a variety of cardiovascular diseases (CVD). In the heart, Ca2+ is essential for maintaining normal cardiac function through the generation of the cardiac action potential and its involvement in excitation contraction coupling. As such, the proteins which regulate Ca2+ cycling and signaling play a vital role in maintaining Ca2+ homeostasis. Changes to the expression levels and function of Ca2+-channels, pumps and associated intracellular handling proteins contribute to altered Ca2+ homeostasis in CVD. The remodeling of Ca2+-handling proteins therefore results in impaired Ca2+ cycling, Ca2+ leak from the sarcoplasmic reticulum and reduced Ca2+ clearance, all of which contributes to increased intracellular Ca2+. Currently, approved treatments for targeting Ca2+ handling dysfunction in CVD are focused on Ca2+ channel blockers. However, whilst Ca2+ channel blockers have been successful in the treatment of some arrhythmic disorders, they are not universally prescribed to heart failure patients owing to their ability to depress cardiac function. Despite the progress in CVD treatments, there remains a clear need for novel therapeutic approaches which are able to reverse pathophysiology associated with heart failure and arrhythmias. Given that heart failure and cardiac arrhythmias are closely associated with altered Ca2+ homeostasis, this review will address the molecular changes to proteins associated with both Ca2+-handling and -signaling; their potential as novel therapeutic targets will be discussed in the context of pre-clinical and, where available, clinical data.
Keywords: arrhythmia; calcium; calcium dysregulation; cardiovascular disease; heart failure.
Copyright © 2020 Njegic, Wilson and Cartwright.
Figures
References
-
- Al-Khatib S. M., Stevenson W. G., Ackerman M. J., Bryant W. J., Callans D. J., Curtis A. B., et al. (2018). 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 72 1677–1749. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

