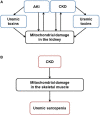
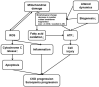
Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia
- PMID: 33013483
- PMCID: PMC7500155
- DOI: 10.3389/fphys.2020.565023
Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia
Abstract
Recently, there has been an increased focus on the influences of mitochondrial dysfunction on various pathologies. Mitochondria are major intracellular organelles with a variety of critical roles, such as adenosine triphosphate production, metabolic modulation, generation of reactive oxygen species, maintenance of intracellular calcium homeostasis, and the regulation of apoptosis. Moreover, mitochondria are attracting attention as a therapeutic target in several diseases. Additionally, a lot of existing agents have been found to have pharmacological effects on mitochondria. This review provides an overview of the mitochondrial change in the kidney and skeletal muscle, which is often complicated with sarcopenia and chronic kidney disease (CKD). Furthermore, the pharmacological effects of therapeutics for CKD on mitochondria are explored.
Keywords: CKD - chronic kidney disease; kidney; mitochondria; sarcopenia; skeletal muscle.
Copyright © 2020 Takemura, Nishi and Inagi.
Figures
References
-
- Allan V. J., Schroer T. A. (1999). Membrane motors. Curr. Opin. Cell Biol. 11 476–482. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

