Efficient Cocaine Degradation by Cocaine Esterase-Loaded Red Blood Cells
- PMID: 33013487
- PMCID: PMC7511699
- DOI: 10.3389/fphys.2020.573492
Efficient Cocaine Degradation by Cocaine Esterase-Loaded Red Blood Cells
Abstract
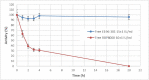
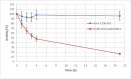
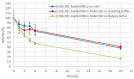
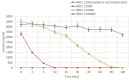
Recombinant bacterial cocaine esterase (CocE) represents a potential protein therapeutic for cocaine use disorder treatment. Unfortunately, the native enzyme was highly unstable and the corresponding mutagenized derivatives, RBP-8000 and E196-301, although improving in vitro thermo-stability and in vivo half-life, were a partial solution to the problem. For cocaine use disorder treatment, an efficient cocaine-metabolizing enzyme with a longer residence time in circulation would be needed. We investigated in vitro the possibility of developing red blood cells (RBCs) loaded with RBP-8000 and E196-301 as a biocompatible system to metabolize cocaine for a longer period of time. RBP 8000 stability within human RBCs is limited (approximately 50% residual activity after 1 h at 37°C) and not different as for the free enzyme, while both free and encapsulated E196-301 showed a greater thermo-stability. By reducing cellular glutathione content during the loading procedure, in order to preserve the disulfide bonds opportunely created to stabilize the enzyme dimer structure, it was possible to produce an encapsulated protein maintaining 100% stability at least after 4 h at 37°C. Moreover, E196-301-loaded RBCs were efficiently able to degrade cocaine in a time- and concentration-dependent manner. The same stability results were obtained when murine RBCs were used paving the way to preclinical investigations. Thus, our in vitro data show that E196-301-loaded RBCs could act as efficient bioreactors in degrading cocaine to non-toxic metabolites to be possibly considered in substance-use disorder treatments. This approach should now be investigated in a preclinical model of cocaine use disorder to evaluate if further protein modifications are needed to further improve long term enzyme stability.
Keywords: E196-301 stability; RBP 8000 stability; cocaine degradation; cocaine esterase; cocaine use disorder; red blood cells as drug delivery system.
Copyright © 2020 Rossi, Pierigè, Agostini, Bigini, Termopoli, Cai, Zheng, Zhan, Landry and Magnani.
Figures



References
-
- Beutler E. (1984a). “Acetylcholinesterase”, in Red Cell Metabolism: A Manual of Biochemical Methods (Orlando: Grune & Stratton, Inc.), 103–104.
-
- Beutler E. (1984b). “Reduced Glutathione (GSH)”, in Red Cell Metabolism: A Manual of Biochemical Methods (Orlando: Grune & Stratton, Inc.), 131–134.
-
- Castro M., Rossi L., Papadatou B., Bracci F., Knafelz D., Ambrosini M. I., et al. (2007). Long-term treatment with autologous red blood cells loaded with dexamethasone 21-phosphate in pediatric patients affected by steroid-dependent Crohn disease. J. Pediatr. Gastroenterol. Nutr. 44 423–426. 10.1097/mpg.0b013e3180320667 - DOI - PubMed
LinkOut - more resources
Full Text Sources

