Regulation of mRNA Stability During Bacterial Stress Responses
- PMID: 33013770
- PMCID: PMC7509114
- DOI: 10.3389/fmicb.2020.02111
Regulation of mRNA Stability During Bacterial Stress Responses
Abstract
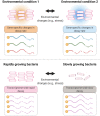
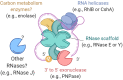
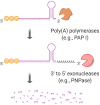
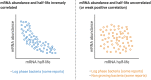
Bacteria have a remarkable ability to sense environmental changes, swiftly regulating their transcriptional and posttranscriptional machinery as a response. Under conditions that cause growth to slow or stop, bacteria typically stabilize their transcriptomes in what has been shown to be a conserved stress response. In recent years, diverse studies have elucidated many of the mechanisms underlying mRNA degradation, yet an understanding of the regulation of mRNA degradation under stress conditions remains elusive. In this review we discuss the diverse mechanisms that have been shown to affect mRNA stability in bacteria. While many of these mechanisms are transcript-specific, they provide insight into possible mechanisms of global mRNA stabilization. To that end, we have compiled information on how mRNA fate is affected by RNA secondary structures; interaction with ribosomes, RNA binding proteins, and small RNAs; RNA base modifications; the chemical nature of 5' ends; activity and concentration of RNases and other degradation proteins; mRNA and RNase localization; and the stringent response. We also provide an analysis of reported relationships between mRNA abundance and mRNA stability, and discuss the importance of stress-associated mRNA stabilization as a potential target for therapeutic development.
Keywords: bacteria; carbon starvation; hypoxia; mRNA degradation; mRNA stability; nutrient starvation; ribonucleic acid; stress response.
Copyright © 2020 Vargas-Blanco and Shell.
Figures



References
-
- Adhya S., Sarkar P., Valenzuela D., Maitra U. (1979). Termination of transcription by Escherichia coli RNA polymerase: influence of secondary structure of RNA transcripts on rho-independent and rho-dependent termination. Proc. Natl. Acad. Sci. U.S.A. 76 1613–1617. 10.1073/pnas.76.4.1613 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

