TCR Redirected T Cells for Cancer Treatment: Achievements, Hurdles, and Goals
- PMID: 33013822
- PMCID: PMC7494743
- DOI: 10.3389/fimmu.2020.01689
TCR Redirected T Cells for Cancer Treatment: Achievements, Hurdles, and Goals
Abstract
Adoptive T cell therapy (ACT) is a rapidly evolving therapeutic approach designed to harness T cell specificity and function to fight diseases. Based on the evidence that T lymphocytes can mediate a potent anti-tumor response, initially ACT solely relied on the isolation, in vitro expansion, and infusion of tumor-infiltrating or circulating tumor-specific T cells. Although effective in a subset of cases, in the first ACT clinical trials several patients experienced disease progression, in some cases after temporary disease control. This evidence prompted researchers to improve ACT products by taking advantage of the continuously evolving gene engineering field and by improving manufacturing protocols, to enable the generation of effective and long-term persisting tumor-specific T cell products. Despite recent advances, several challenges, including prioritization of antigen targets, identification, and optimization of tumor-specific T cell receptors, in the development of tools enabling T cells to counteract the immunosuppressive tumor microenvironment, still need to be faced. This review aims at summarizing the major achievements, hurdles and possible solutions designed to improve the ACT efficacy and safety profile in the context of liquid and solid tumors.
Keywords: TCR - T cell receptor; adoptive T cell immunotherapy; cancer immunoediting; cancer immunotherapy; genetic engineering.
Copyright © 2020 Manfredi, Cianciotti, Potenza, Tassi, Noviello, Biondi, Ciceri, Bonini and Ruggiero.
Figures
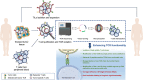
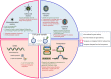
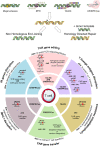

References
-
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. (2010) 116:4099–102. 10.1182/blood-2010-04-281931 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

