Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia
- PMID: 33013837
- PMCID: PMC7462000
- DOI: 10.3389/fimmu.2020.01864
Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia
Abstract
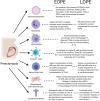
Preeclampsia is a complex cardiovascular disorder of pregnancy with underlying multifactorial pathogeneses; however, its etiology is not fully understood. It is characterized by the new onset of maternal hypertension after 20 weeks of gestation, accompanied by proteinuria, maternal organ damage, and/or uteroplacental dysfunction. Preeclampsia can be subdivided into early- and late-onset phenotypes (EOPE and LOPE), diagnosed before 34 weeks or from 34 weeks of gestation, respectively. Impaired placental development in early pregnancy and subsequent growth restriction is often associated with EOPE, while LOPE is associated with maternal endothelial dysfunction. The innate immune system plays an essential role in normal progression of physiological pregnancy and fetal development. However, inappropriate or excessive activation of this system can lead to placental dysfunction or poor maternal vascular adaptation and contribute to the development of preeclampsia. This review aims to comprehensively outline the mechanisms of key innate immune cells including macrophages, neutrophils, natural killer (NK) cells, and innate B1 cells, in normal physiological pregnancy, EOPE and LOPE. The roles of the complement system, syncytiotrophoblast extracellular vesicles and mesenchymal stem cells (MSCs) are also discussed in the context of innate immune system regulation and preeclampsia. The outlined molecular mechanisms, which represent potential therapeutic targets, and associated emerging treatments, are evaluated as treatments for preeclampsia. Therefore, by addressing the current understanding of innate immunity in the pathogenesis of EOPE and LOPE, this review will contribute to the body of research that could lead to the development of better diagnosis, prevention, and treatment strategies. Importantly, it will delineate the differences in the mechanisms of the innate immune system in two different types of preeclampsia, which is necessary for a more personalized approach to the monitoring and treatment of affected women.
Keywords: early-onset preeclampsia; immune cells; inflammation; innate immunity; late-onset preeclampsia; preeclampsia; pregnancy.
Copyright © 2020 Aneman, Pienaar, Suvakov, Simic, Garovic and McClements.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

