Quantification of Factor H Mediated Self vs. Non-self Discrimination by Mathematical Modeling
- PMID: 33013842
- PMCID: PMC7493836
- DOI: 10.3389/fimmu.2020.01911
Quantification of Factor H Mediated Self vs. Non-self Discrimination by Mathematical Modeling
Abstract
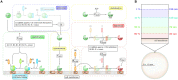
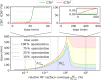
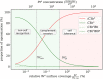
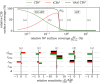
The complement system is part of the innate immune system and plays an important role in the host defense against infectious pathogens. One of the main effects is the opsonization of foreign invaders and subsequent uptake by phagocytosis. Due to the continuous default basal level of active complement molecules, a tight regulation is required to protect the body's own cells (self cells) from opsonization and from complement damage. A major complement regulator is Factor H, which is recruited from the fluid phase and attaches to cell surfaces where it effectively controls complement activation. Besides self cells, pathogens also have the ability to bind Factor H; they can thus escape opsonization and phagocytosis causing severe infections. In order to advance our understanding of the opsonization process at a quantitative level, we developed a mathematical model for the dynamics of the complement system-termed DynaCoSys model-that is based on ordinary differential equations for cell surface-bound molecules and on partial differential equations for concentration profiles of the fluid phase molecules in the environment of cells. This hybrid differential equation approach allows to model the complement cascade focusing on the role of active C3b in the fluid phase and on the cell surface as well as on its inactivation on the cell surface. The DynaCoSys model enables us to quantitatively predict the conditions under which Factor H mediated complement evasion occurs. Furthermore, investigating the quantitative impact of model parameters by a sensitivity analysis, we identify the driving processes of complement activation and regulation in both the self and non-self regime. The two regimes are defined by a critical Factor H concentration on the cell surface and we use the model to investigate the differential impact of complement model parameters on this threshold value. The dynamic modeling on the surface of pathogens are further relevant to understand pathophysiological situations where Factor H mutants and defective Factor H binding to target surfaces results in pathophysiology such as renal and retinal disease. In the future, this DynaCoSys model will be extended to also enable evaluating treatment strategies of complement-related diseases.
Keywords: complement regulator factor H; complement system; hybrid differential equation approach; immune evasion; mathematical modeling; non-self recognition; self-tolerance.
Copyright © 2020 Tille, Lehnert, Zipfel and Figge.
Figures



References
-
- Flaherty D. editor. Complement. In: Immunology for Pharmacy. Saint Louis, MO: Mosby; (2012). p. 87–96. 10.1016/B978-0-323-06947-2.10011-2 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

