Update on Transplacental Transfer of IgG Subclasses: Impact of Maternal and Fetal Factors
- PMID: 33013843
- PMCID: PMC7516031
- DOI: 10.3389/fimmu.2020.01920
Update on Transplacental Transfer of IgG Subclasses: Impact of Maternal and Fetal Factors
Abstract
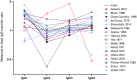
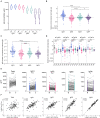
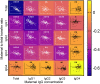
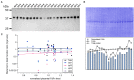
Transplacental antibody transfer from mother to fetus provides protection from infection in the first weeks of life, and the four different subclasses of IgG (IgG1, IgG2, IgG3, and IgG4) have diverse roles in protection against infection. In this study, we evaluated concentrations and transplacental transfer ratios of the IgG subclasses in a healthy UK-based cohort of mother-cord pairs, and investigated associations with maternal, obstetric, and fetal factors. In agreement with previous studies, we found a strong association between maternal and cord IgG for all subclasses. We report a transfer efficiency hierarchy of IgG1>IgG3>IgG4=IgG2 in our study population, and our review of the literature demonstrates that there is no consensus in the hierarchy of subclass transfer, despite the commonly made statement that the order is IgG1>IgG4>IgG3>IgG2. We report additional data regarding negative associations between elevated maternal IgG concentrations and maternal/cord transfer ratios, finding an effect on IgG1, IgG2, and IgG3 subclasses. Levels of IgG subclasses were the same between venous and arterial blood samples from the umbilical cord, but there was a significantly higher level of total IgG in arterial blood. We found no correlation between placental FcRn protein levels and IgG transfer in our cohort, suggesting that IgG is the main determinant of observed differences in transplacental transfer ratios at term. Neonatal IgG1 and IgG4 levels were increased with later gestation at delivery, independent of any increase in transplacental transfer, indicating that the benefit of later gestation is through accumulation of these subclasses in the fetus. Neonatal IgG2 levels and transfer ratios were reduced in rhesus-negative pregnancies, suggesting that administered anti-D antibodies may compete for transplacental transfer of this subclass. Maternal influenza vaccination resulted in elevated maternal and neonatal levels of IgG4, whereas maternal Tdap vaccination had no impact on neonatal levels of the subclasses, nor transfer. However, within Tdap vaccinated pregnancies, later gestation at Tdap vaccination was associated with higher transplacental transfer. Our study provides information regarding levels and transfer of IgG subclasses in healthy term pregnancies and demonstrates the importance of recording detailed clinical information in studies of antibody transfer, including parity, ethnicity, and timing of maternal vaccine delivery.
Keywords: IgG; antibody; immunology; infection; maternal vaccination; neonatal; placenta; pregnancy.
Copyright © 2020 Clements, Rice, Vamvakas, Barnett, Barnes, Donaldson, Jones, Kampmann and Holder.
Figures

Similar articles
-
Placental transfer of IgG subclasses in a Japanese population.Pediatr Int. 2000 Aug;42(4):337-42. doi: 10.1046/j.1442-200x.2000.01245.x. Pediatr Int. 2000. PMID: 10986861
-
Associations between an IgG3 polymorphism in the binding domain for FcRn, transplacental transfer of malaria-specific IgG3, and protection against Plasmodium falciparum malaria during infancy: A birth cohort study in Benin.PLoS Med. 2017 Oct 9;14(10):e1002403. doi: 10.1371/journal.pmed.1002403. eCollection 2017 Oct. PLoS Med. 2017. PMID: 28991911 Free PMC article.
-
Efficiency of placental transfer of vaccine-elicited antibodies relative to prenatal Tdap vaccination status.Vaccine. 2020 Jun 26;38(31):4869-4876. doi: 10.1016/j.vaccine.2020.05.036. Epub 2020 May 29. Vaccine. 2020. PMID: 32482459 Free PMC article.
-
The influence of maternal immunization on infant immune responses.J Comp Pathol. 2007 Jul;137 Suppl 1:S16-9. doi: 10.1016/j.jcpa.2007.04.006. Epub 2007 Jun 5. J Comp Pathol. 2007. PMID: 17553516 Review.
-
Transplacental transport of IgG antibodies to preterm infants: a review of the literature.Early Hum Dev. 2011 Feb;87(2):67-72. doi: 10.1016/j.earlhumdev.2010.11.003. Epub 2010 Nov 30. Early Hum Dev. 2011. PMID: 21123010 Review.
Cited by
-
Immunoglobulin G passive transfer from mothers to infants: total IgG, IgG subclasses and specific antipneumococcal IgG in 6-week Malawian infants exposed or unexposed to HIV.BMC Infect Dis. 2022 Apr 5;22(1):342. doi: 10.1186/s12879-022-07335-0. BMC Infect Dis. 2022. PMID: 35382749 Free PMC article.
-
FcRn-enhancing mutations lead to increased and prolonged levels of the HIV CCR5-blocking monoclonal antibody leronlimab in the fetuses and newborns of pregnant rhesus macaques.MAbs. 2024 Jan-Dec;16(1):2406788. doi: 10.1080/19420862.2024.2406788. Epub 2024 Sep 26. MAbs. 2024. PMID: 39324549 Free PMC article.
-
Dissecting Fc signatures of protection in neonates following maternal influenza vaccination in a placebo-controlled trial.Cell Rep. 2022 Feb 8;38(6):110337. doi: 10.1016/j.celrep.2022.110337. Cell Rep. 2022. PMID: 35139373 Free PMC article. Clinical Trial.
-
Maternal vaccination against RSV can substantially reduce childhood mortality in low-income and middle-income countries: A mathematical modeling study.Vaccine X. 2023 Sep 1;15:100379. doi: 10.1016/j.jvacx.2023.100379. eCollection 2023 Dec. Vaccine X. 2023. PMID: 37711264 Free PMC article.
-
Development of Porcine Monoclonal Antibodies with In Vitro Neutralizing Activity against Classical Swine Fever Virus from C-Strain E2-Specific Single B Cells.Viruses. 2023 Mar 28;15(4):863. doi: 10.3390/v15040863. Viruses. 2023. PMID: 37112845 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

