The Immunologic Effect of Early Intravenous Two and Four Gram Bolus Dosing of Tranexamic Acid Compared to Placebo in Patients With Severe Traumatic Bleeding (TAMPITI): A Randomized, Double-Blind, Placebo-Controlled, Single-Center Trial
- PMID: 33013880
- PMCID: PMC7506112
- DOI: 10.3389/fimmu.2020.02085
The Immunologic Effect of Early Intravenous Two and Four Gram Bolus Dosing of Tranexamic Acid Compared to Placebo in Patients With Severe Traumatic Bleeding (TAMPITI): A Randomized, Double-Blind, Placebo-Controlled, Single-Center Trial
Abstract
Background: The hemostatic properties of tranexamic acid (TXA) are well described, but the immunological effects of TXA administration after traumatic injury have not been thoroughly examined. We hypothesized TXA would reduce monocyte activation in bleeding trauma patients with severe injury.
Methods: This was a single center, double-blinded, randomized controlled trial (RCT) comparing placebo to a 2 g or 4 g intravenous TXA bolus dose in trauma patients with severe injury. Fifty patients were randomized into each study group. The primary outcome was a reduction in monocyte activation as measured by human leukocyte antigen-DR isotype (HLA-DR) expression on monocytes 72 h after TXA administration. Secondary outcomes included kinetic assessment of immune and hemostatic phenotypes within the 72 h window post-TXA administration.
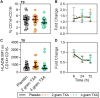
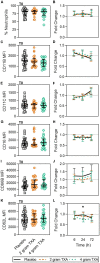
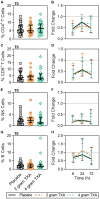
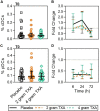
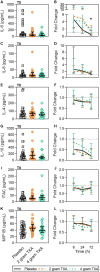
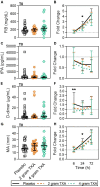
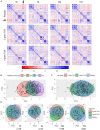
Results: The trial occurred between March 2016 and September 2017, when data collection ended. 149 patients were analyzed (placebo, n = 50; 2 g TXA, n = 49; 4 g TXA, n = 50). The fold change in HLA-DR expression on monocytes [reported as median (Q1-Q3)] from pre-TXA to 72 h post-TXA was similar between placebo [0.61 (0.51-0.82)], 2 g TXA [0.57 (0.47-0.75)], and 4 g TXA [0.57 (0.44-0.89)] study groups (p = 0.82). Neutrophil CD62L expression was reduced in the 4 g TXA group [fold change: 0.73 (0.63-0.97)] compared to the placebo group [0.97 (0.78-1.10)] at 24 h post-TXA (p = 0.034). The fold decrease in plasma IL-6 was significantly less in the 4 g TXA group [1.36 (0.87-2.42)] compared to the placebo group [0.46 (0.19-1.69)] at 72 h post-TXA (p = 0.028). There were no differences in frequencies of myeloid or lymphoid populations or in classical complement activation at any of the study time points.
Conclusion: In trauma patients with severe injury, 4 g intravenous bolus dosing of TXA has minimal immunomodulatory effects with respect to leukocyte phenotypes and circulating cytokine levels.
Clinical trial registration: www.ClinicalTrials.gov, identifier NCT02535949.
Keywords: hemostasis; immunology; monocyte activation; tranexamic acid; trauma.
Copyright © 2020 Spinella, Thomas, Turnbull, Fuchs, Bochicchio, Schuerer, Reese, Coleoglou Centeno, Horn, Baty, Shea, Meledeo, Pusateri, Levy, Cap and Bochicchio.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

