Reconstituting NK Cells After Allogeneic Stem Cell Transplantation Show Impaired Response to the Fungal Pathogen Aspergillus fumigatus
- PMID: 33013893
- PMCID: PMC7511764
- DOI: 10.3389/fimmu.2020.02117
Reconstituting NK Cells After Allogeneic Stem Cell Transplantation Show Impaired Response to the Fungal Pathogen Aspergillus fumigatus
Abstract
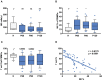
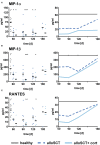
Delayed natural killer (NK) cell reconstitution after allogeneic stem cell transplantation (alloSCT) is associated with a higher risk of developing invasive aspergillosis. The interaction of NK cells with the human pathogen Aspergillus (A.) fumigatus is mediated by the fungal recognition receptor CD56, which is relocated to the fungal interface after contact. Blocking of CD56 signaling inhibits the fungal mediated chemokine secretion of MIP-1α, MIP-1β, and RANTES and reduces cell activation, indicating a functional role of CD56 in fungal recognition. We collected peripheral blood from recipients of an allograft at defined time points after alloSCT (day 60, 90, 120, 180). NK cells were isolated, directly challenged with live A. fumigatus germ tubes, and cell function was analyzed and compared to healthy age and gender-matched individuals. After alloSCT, NK cells displayed a higher percentage of CD56brightCD16dim cells throughout the time of blood collection. However, CD56 binding and relocalization to the fungal contact side were decreased. We were able to correlate this deficiency to the administration of corticosteroid therapy that further negatively influenced the secretion of MIP-1α, MIP-1β, and RANTES. As a consequence, the treatment of healthy NK cells ex vivo with corticosteroids abrogated chemokine secretion measured by multiplex immunoassay. Furthermore, we analyzed NK cells regarding their actin cytoskeleton by Structured Illumination Microscopy (SIM) and flow cytometry and demonstrate an actin dysfunction of NK cells shown by reduced F-actin content after fungal co-cultivation early after alloSCT. This dysfunction remains until 180 days post-alloSCT, concluding that further actin-dependent cellular processes may be negatively influenced after alloSCT. To investigate the molecular pathomechansism, we compared CD56 receptor mobility on the plasma membrane of healthy and alloSCT primary NK cells by single-molecule tracking. The results were very robust and reproducible between tested conditions which point to a different molecular mechanism and emphasize the importance of proper CD56 mobility.
Keywords: Aspergillus fumigatus; CCL3; CCL4; CCL5; corticosteroids; natural killer cell; stem cell transplantation.
Copyright © 2020 Weiss, Schlegel, Terpitz, Weber, Linde, Schmitt, Hünniger, Marischen, Gamon, Bauer, Löffler, Kurzai, Morton, Sauer, Einsele and Loeffler.
Figures







Similar articles
-
CD56 Is a Pathogen Recognition Receptor on Human Natural Killer Cells.Sci Rep. 2017 Jul 21;7(1):6138. doi: 10.1038/s41598-017-06238-4. Sci Rep. 2017. PMID: 28733594 Free PMC article.
-
Human NK Cells Develop an Exhaustion Phenotype During Polar Degranulation at the Aspergillus fumigatus Hyphal Synapse.Front Immunol. 2018 Oct 22;9:2344. doi: 10.3389/fimmu.2018.02344. eCollection 2018. Front Immunol. 2018. PMID: 30405602 Free PMC article.
-
CD56-mediated activation of human natural killer cells is triggered by Aspergillus fumigatus galactosaminogalactan.PLoS Pathog. 2024 Jun 18;20(6):e1012315. doi: 10.1371/journal.ppat.1012315. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38889192 Free PMC article.
-
Role of chemokines in the biology of natural killer cells.Curr Top Microbiol Immunol. 2010;341:37-58. doi: 10.1007/82_2010_20. Curr Top Microbiol Immunol. 2010. PMID: 20369317 Review.
-
Role of chemokines in the biology of natural killer cells.J Leukoc Biol. 2002 Feb;71(2):173-83. J Leukoc Biol. 2002. PMID: 11818437 Review.
Cited by
-
In vitro infection models to study fungal-host interactions.FEMS Microbiol Rev. 2021 Sep 8;45(5):fuab005. doi: 10.1093/femsre/fuab005. FEMS Microbiol Rev. 2021. PMID: 33524102 Free PMC article. Review.
-
Defective antifungal immunity in patients with COVID-19.Front Immunol. 2022 Nov 30;13:1080822. doi: 10.3389/fimmu.2022.1080822. eCollection 2022. Front Immunol. 2022. PMID: 36531987 Free PMC article. Review.
-
Subdiffraction-resolution fluorescence imaging of immunological synapse formation between NK cells and A. fumigatus by expansion microscopy.Commun Biol. 2021 Oct 4;4(1):1151. doi: 10.1038/s42003-021-02669-y. Commun Biol. 2021. PMID: 34608260 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

