CD226 Deletion Reduces Type 1 Diabetes in the NOD Mouse by Impairing Thymocyte Development and Peripheral T Cell Activation
- PMID: 33013915
- PMCID: PMC7500101
- DOI: 10.3389/fimmu.2020.02180
CD226 Deletion Reduces Type 1 Diabetes in the NOD Mouse by Impairing Thymocyte Development and Peripheral T Cell Activation
Abstract
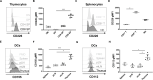
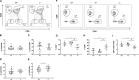
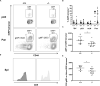
The costimulatory molecule CD226 is highly expressed on effector/memory T cells and natural killer cells. Costimulatory signals received by T cells can impact both central and peripheral tolerance mechanisms. Genetic polymorphisms in CD226 have been associated with susceptibility to type 1 diabetes and other autoimmune diseases. We hypothesized that genetic deletion of Cd226 in the non-obese diabetic (NOD) mouse would impact type 1 diabetes incidence by altering T cell activation. CD226 knockout (KO) NOD mice displayed decreased disease incidence and insulitis in comparison to wild-type (WT) controls. Although female CD226 KO mice had similar levels of sialoadenitis as WT controls, male CD226 KO mice showed protection from dacryoadenitis. Moreover, CD226 KO T cells were less capable of adoptively transferring disease compared to WT NOD T cells. Of note, CD226 KO mice demonstrated increased CD8+ single positive (SP) thymocytes, leading to increased numbers of CD8+ T cells in the spleen. Decreased percentages of memory CD8+CD44+CD62L- T cells were observed in the pancreatic lymph nodes of CD226 KO mice. Intriguingly, CD8+ T cells in CD226 KO mice showed decreased islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)-tetramer and CD5 staining, suggesting reduced T cell receptor affinity for this immunodominant antigen. These data support an important role for CD226 in type 1 diabetes development by modulating thymic T cell selection as well as impacting peripheral memory/effector CD8+ T cell activation and function.
Keywords: CD226; CD8+ T cells; Sjögren’s disease; costimulatory receptors; type 1 diabetes.
Copyright © 2020 Shapiro, Yeh, Longfield, Gallagher, Infante, Wellford, Posgai, Atkinson, Campbell-Thompson, Lieberman, Serreze, Geurts, Chen and Brusko.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

