Effective Nanoparticle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep
- PMID: 33013917
- PMCID: PMC7509486
- DOI: 10.3389/fimmu.2020.02183
Effective Nanoparticle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep
Abstract
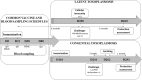
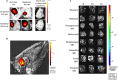
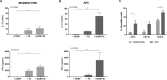
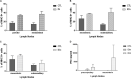
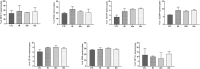
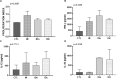
Toxoplasma gondii is a parasitic protozoan of worldwide distribution, able to infect all warm-blooded animals, but particularly sheep. Primary infection in pregnant sheep leads to millions of abortions and significant economic losses for the livestock industry. Moreover, infected animals constitute the main parasitic reservoir for humans. Therefore, the development of a One-health vaccine seems the best prevention strategy. Following earlier work, a vaccine constituted of total extract of Toxoplasma gondii proteins (TE) associated with maltodextrin nanoparticles (DGNP) was developed in rodents. In this study we evaluated the ability of this vaccine candidate to protect against latent and congenital toxoplasmosis in sheep. After two immunizations by either intranasal or intradermal route, DGNP/TE vaccine generated specific Th1-cellular immune response, mediated by APC-secretion of IFN-γ and IL-12. Secretion of IL-10 appeared to regulate this Th1 response for intradermally vaccinated sheep but was absent in intranasally-vaccinated animals. Finally, protection against latent toxoplasmosis and transplacental transmission were explored. Intranasal vaccination led to a marked decrease of brain cysts compared with the non-vaccinated group. This DGNP/TE vaccine administered intranasally conferred a high level of protection against latent toxoplasmosis and its transplacental transmission in sheep, highlighting the potential for development of such a vaccine for studies in other species.
Keywords: adjuvant free; nanoparticle; nasal vaccination; one-health approach; ovine toxoplasmosis.
Copyright © 2020 Ducournau, Moiré, Carpentier, Cantin, Herkt, Lantier, Betbeder and Dimier-Poisson.
Figures

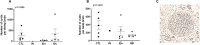
References
-
- Dubey JP, Beattie CP. Toxoplasmosis of Animals and Man. Boca Raton, FL: CRC Press; (1988).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

