Red Light Controls Adventitious Root Regeneration by Modulating Hormone Homeostasis in Picea abies Seedlings
- PMID: 33014006
- PMCID: PMC7509059
- DOI: 10.3389/fpls.2020.586140
Red Light Controls Adventitious Root Regeneration by Modulating Hormone Homeostasis in Picea abies Seedlings
Abstract
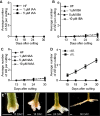
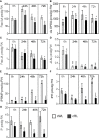
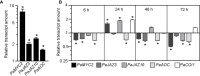
Vegetative propagation relies on the capacity of plants to regenerate de novo adventitious roots (ARs), a quantitative trait controlled by the interaction of endogenous factors, such as hormones and environmental cues among which light plays a central role. However, the physiological and molecular components mediating light cues during AR initiation (ARI) remain largely elusive. Here, we explored the role of red light (RL) on ARI in de-rooted Norway spruce seedlings. We combined investigation of hormone metabolism and gene expression analysis to identify potential signaling pathways. We also performed extensive anatomical characterization to investigate ARI at the cellular level. We showed that in contrast to white light, red light promoted ARI likely by reducing jasmonate (JA) and JA-isoleucine biosynthesis and repressing the accumulation of isopentyl-adenine-type cytokinins. We demonstrated that exogenously applied JA and/or CK inhibit ARI in a dose-dependent manner and found that they possibly act in the same pathway. The negative effect of JA on ARI was confirmed at the histological level. We showed that JA represses the early events of ARI. In conclusion, RL promotes ARI by repressing the accumulation of the wound-induced phytohormones JA and CK.
Keywords: Picea abies; adventitious roots; auxin; conifers; cytokinins; jasmonate; red light; root development.
Copyright © 2020 Alallaq, Ranjan, Brunoni, Novák, Lakehal and Bellini.
Figures



References
-
- Abarca D., Díaz-Sala C. (2009). “Adventitious root formation in conifers,” in Adventitious Root Formation of Forest Trees and Horticultural Plants – from Genes to Applications. Eds. Niemi K., Scagel C. (Kerala, India: Research Signpost Publishers; ).
-
- Agulló-Antón M.Á., Sánchez-Bravo J., Acosta M., Druege U. (2011). Auxins or Sugars: What Makes the Difference in the Adventitious Rooting of Stored Carnation Cuttings? J. Plant Growth Regul. 30, 100–113. 10.1007/s00344-010-9174-8 - DOI
-
- Baque M. A., Hahn E.-J., Paek K.-Y. (2010). Induction mechanism of adventitious root from leaf explants of Morinda citrifolia as affected by auxin and light quality. Vitr. Cell. Dev. Biol. - Plant 46, 71–80. 10.1007/s11627-009-9261-3 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

