High Cysteine Membrane Proteins (HCMPs) Are Up-Regulated During Giardia-Host Cell Interactions
- PMID: 33014015
- PMCID: PMC7461913
- DOI: 10.3389/fgene.2020.00913
High Cysteine Membrane Proteins (HCMPs) Are Up-Regulated During Giardia-Host Cell Interactions
Abstract
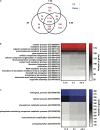
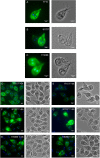
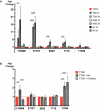
Giardia intestinalis colonizes the upper small intestine of humans and animals, causing the diarrheal disease giardiasis. This unicellular eukaryotic parasite is not invasive but it attaches to the surface of small intestinal epithelial cells (IECs), disrupting the epithelial barrier. Here, we used an in vitro model of the parasite's interaction with host IECs (differentiated Caco-2 cells) and RNA sequencing (RNAseq) to identify differentially expressed genes (DEGs) in Giardia, which might relate to the establishment of infection and disease induction. Giardia trophozoites interacted with differentiated Caco-2 cells for 1.5, 3, and 4.5 h and at each time point, 61, 89, and 148 parasite genes were up-regulated more than twofold, whereas 209, 265, and 313 parasite genes were down-regulated more than twofold. The most abundant DEGs encode hypothetical proteins and members of the High Cysteine Membrane Protein (HCMP) family. Among the up-regulated genes we also observed proteins associated with proteolysis, cellular redox balance, as well as lipid and nucleic acid metabolic pathways. In contrast, genes encoding kinases, regulators of the cell cycle and arginine metabolism and cytoskeletal proteins were down-regulated. Immunofluorescence imaging of selected, up-regulated HCMPs, using C-terminal HA-tagging, showed localization to the plasma membrane and peripheral vesicles (PVs). The expression of the HCMPs was affected by histone acetylation and free iron-levels. In fact, the latter was shown to regulate the expression of many putative giardial virulence factors in subsequent RNAseq experiments. We suggest that the plasma membrane localized and differentially expressed HCMPs play important roles during Giardia-host cell interactions.
Keywords: RNAseq; chromatin; diarrhea; protozoa; small intestine.
Copyright © 2020 Peirasmaki, Ma’ayeh, Xu, Ferella, Campos, Liu and Svärd.
Figures
Similar articles
-
Dual RNA Sequencing Reveals Key Events When Different Giardia Life Cycle Stages Interact With Human Intestinal Epithelial Cells In Vitro.Front Cell Infect Microbiol. 2022 Apr 27;12:862211. doi: 10.3389/fcimb.2022.862211. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35573800 Free PMC article.
-
Transcriptional changes in Giardia during host-parasite interactions.Int J Parasitol. 2011 Mar;41(3-4):277-85. doi: 10.1016/j.ijpara.2010.09.011. Epub 2010 Nov 11. Int J Parasitol. 2011. PMID: 21074536
-
Transcriptomic and proteomic analyses of Giardia intestinalis: Intestinal epithelial cell interactions.Adv Parasitol. 2020;107:139-171. doi: 10.1016/bs.apar.2019.11.002. Epub 2019 Nov 25. Adv Parasitol. 2020. PMID: 32122528 Review.
-
Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: The impact on host cells.PLoS Negl Trop Dis. 2017 Dec 11;11(12):e0006120. doi: 10.1371/journal.pntd.0006120. eCollection 2017 Dec. PLoS Negl Trop Dis. 2017. PMID: 29228011 Free PMC article.
-
Giardia duodenalis: Role of secreted molecules as virulent factors in the cytotoxic effect on epithelial cells.Adv Parasitol. 2019;106:129-169. doi: 10.1016/bs.apar.2019.07.003. Epub 2019 Aug 20. Adv Parasitol. 2019. PMID: 31630757 Review.
Cited by
-
Stem-Loop Structures in Iron-Regulated mRNAs of Giardia duodenalis.Int J Environ Res Public Health. 2023 Feb 17;20(4):3556. doi: 10.3390/ijerph20043556. Int J Environ Res Public Health. 2023. PMID: 36834255 Free PMC article.
-
Characterization of Metronidazole-Resistant Giardia intestinalis Lines by Comparative Transcriptomics and Proteomics.Front Microbiol. 2022 Feb 10;13:834008. doi: 10.3389/fmicb.2022.834008. eCollection 2022. Front Microbiol. 2022. PMID: 35222342 Free PMC article.
-
Giardia duodenalis Virulence - "To Be, or Not To Be".Curr Trop Med Rep. 2021;8(4):246-256. doi: 10.1007/s40475-021-00248-z. Epub 2021 Oct 21. Curr Trop Med Rep. 2021. PMID: 34697581 Free PMC article. Review.
-
A core UPS molecular complement implicates unique endocytic compartments at the parasite-host interface in Giardia lamblia.Virulence. 2023 Dec;14(1):2174288. doi: 10.1080/21505594.2023.2174288. Virulence. 2023. PMID: 36730629 Free PMC article.
-
Trophozoite fitness dictates the intestinal epithelial cell response to Giardia intestinalis infection.PLoS Pathog. 2023 May 4;19(5):e1011372. doi: 10.1371/journal.ppat.1011372. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37141303 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

