The Retinal Inner Plexiform Synaptic Layer Mirrors Grey Matter Thickness of Primary Visual Cortex with Increased Amyloid β Load in Early Alzheimer's Disease
- PMID: 33014034
- PMCID: PMC7525303
- DOI: 10.1155/2020/8826087
The Retinal Inner Plexiform Synaptic Layer Mirrors Grey Matter Thickness of Primary Visual Cortex with Increased Amyloid β Load in Early Alzheimer's Disease
Abstract
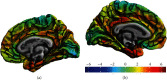
The retina may serve as putative window into neuropathology of synaptic loss in Alzheimer's disease (AD). Here, we investigated synapse-rich layers versus layers composed by nuclei/cell bodies in an early stage of AD. In addition, we examined the associations between retinal changes and molecular and structural markers of cortical damage. We recruited 20 AD patients and 17 healthy controls (HC). Combining optical coherence tomography (OCT), magnetic resonance (MR), and positron emission tomography (PET) imaging, we measured retinal and primary visual cortex (V1) thicknesses, along with V1 amyloid β (Aβ) retention ([11C]-PiB PET tracer) and neuroinflammation ([11C]-PK11195 PET tracer). We found that V1 showed increased amyloid-binding potential, in the absence of neuroinflammation. Although thickness changes were still absent, we identified a positive association between the synapse-rich inner plexiform layer (IPL) and V1 in AD. This retinocortical interplay might reflect changes in synaptic function resulting from Aβ deposition, contributing to early visual loss.
Copyright © 2020 Lília Jorge et al.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures
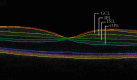


References
-
- Avila J., Pallas N., Bolós M., Sayas C. L., Hernandez F. Intracellular and extracellular microtubule associated protein tau as a therapeutic target in Alzheimer disease and other tauopathies. Expert Opinion on Therapeutic Targets. 2015;20(6):653–661. doi: 10.1517/14728222.2016.1131269. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

