Effect of Combined Live Probiotics Alleviating the Gastrointestinal Symptoms of Functional Bowel Disorders
- PMID: 33014039
- PMCID: PMC7519468
- DOI: 10.1155/2020/4181748
Effect of Combined Live Probiotics Alleviating the Gastrointestinal Symptoms of Functional Bowel Disorders
Abstract
Objective: Changes of the gut microbiota are related to the pathogenesis of functional bowel disorders (FBDs), and probiotic supplementation may be an effective treatment option. Therefore, we aimed to investigate the effect of combined live probiotics on the gastrointestinal symptoms of FBDs via altering the gut microbiota.
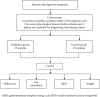
Methods: Patients with the gastrointestinal symptoms of FBDs attending the Outpatient Department, from July to November 2019, were recruited. After the bowel preparation with polyethylene glycol electrolyte powder and colonoscopy, patients with normal result of colonoscopy were randomly divided into the probiotics group and control group. Patients in the probiotics group were prescribed with combined live Bacillus subtilis and Enterococcus faecium enteric-coated capsules for 4 weeks. Small intestinal bacteria overgrowth (SIBO) was measured by lactulose hydrogen breath test, and the microbial DNA was extracted from the fecal samples and the bacteria were classified by 16S rDNA gene amplicon sequencing.
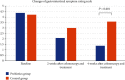
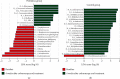
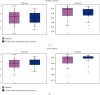
Results: Twenty-five patients of each group were recruited, and there was no significant difference between the probiotics and control groups on baseline gastrointestinal symptom rating scale (GSRS), positive rate of SIBO, and relative abundances of the gut microbiota at the phylum level. After 4 weeks of treatment, the values of the probiotics and control groups were as follows: GSRS 1.4 ± 1.4 and 3.6 ± 1.6 and positive rate of SIBO 28.0% and 56.0%, respectively. The median relative abundances of the gut microbiota were 1.01% and 5.03% Actinobacteria and 43.80% and 35.17% Bacteroidetes at the phylum level; 0.76% and 3.29% Bifidobacterium, 0.13% and 0.89% Cillinsella, 0.03% and 0.01% Enterococcus, 0.18% and 0.36% Lachnospiraceae, 0.10% and 0.16% Ruminococcus torques group, 1.31% and 2.44% Blautia, and 0.83% and 2.02% Fusicatenibacter at the genus level (P < 0.05), respectively.
Conclusion: Combined live probiotic supplementation after the bowel preparation can alter the gut microbiota, decontaminate SIBO, and alleviate the gastrointestinal symptoms of FBDs. This trial is registered with ChiCTR1900026472.
Copyright © 2020 Jin Shi et al.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
LinkOut - more resources
Full Text Sources

