Differentiation Potential of Early- and Late-Passage Adipose-Derived Mesenchymal Stem Cells Cultured under Hypoxia and Normoxia
- PMID: 33014073
- PMCID: PMC7519987
- DOI: 10.1155/2020/8898221
Differentiation Potential of Early- and Late-Passage Adipose-Derived Mesenchymal Stem Cells Cultured under Hypoxia and Normoxia
Abstract
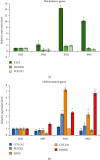
With an increasing focus on the large-scale expansion of mesenchymal stem cells (MSCs) required for clinical applications for the treatment of joint and bone diseases such as osteoarthritis, the optimisation of conditions for in vitro MSC expansion requires careful consideration to maintain native MSC characteristics. Physiological parameters such as oxygen concentration, media constituents, and passage numbers influence the properties of MSCs and may have major impact on their therapeutic potential. Cells grown under hypoxic conditions have been widely documented in clinical use. Culturing MSCs on large scale requires bioreactor culture; however, it is challenging to maintain low oxygen and other physiological parameters over several passages in large bioreactor vessels. The necessity to scale up the production of cells in vitro under normoxia may affect important attributes of MSCs. For these reasons, our study investigated the effects of normoxic and hypoxic culture condition on early- and late-passage adipose-derived MSCs. We examined effect of each condition on the expression of key stem cell marker genes POU5F1, NANOG, and KLF4, as well as differentiation genes RUNX2, COL1A1, SOX9, COL2A1, and PPARG. We found that expression levels of stem cell marker genes and osteogenic and chondrogenic genes were higher in normoxia compared to hypoxia. Furthermore, expression of these genes reduced with passage number, with the exception of PPARG, an adipose differentiation marker, possibly due to the adipose origin of the MSCs. We confirmed by flow cytometry the presence of cell surface markers CD105, CD73, and CD90 and lack of expression of CD45, CD34, CD14, and CD19 across all conditions. Furthermore, in vitro differentiation confirmed that both early- and late-passage adipose-derived MSCs grown in hypoxia or normoxia could differentiate into chondrogenic and osteogenic cell types. Our results demonstrate that the minimal standard criteria to define MSCs as suitable for laboratory-based and preclinical studies can be maintained in early- or late-passage MSCs cultured in hypoxia or normoxia. Therefore, any of these culture conditions could be used when scaling up MSCs in bioreactors for allogeneic clinical applications or tissue engineering for the treatment of joint and bone diseases such as osteoarthritis.
Copyright © 2020 Ashley G. Zhao et al.
Conflict of interest statement
Authors have no conflicts of interest.
Figures



References
-
- Samsonraj R. M., Raghunath M., Nurcombe V., Hui J. H., van Wijnen A. J., Cool S. M. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Translational Medicine. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

