Assessing the benefits and harms of direct oral anticoagulants in patients with cancer for the prophylaxis and treatment of venous thromboembolism: a systematic review and meta-analysis
- PMID: 33014133
- PMCID: PMC7498277
- DOI: 10.3332/ecancer.2020.1091
Assessing the benefits and harms of direct oral anticoagulants in patients with cancer for the prophylaxis and treatment of venous thromboembolism: a systematic review and meta-analysis
Abstract
Background: Direct oral anticoagulants (DOACs) have recently been tested in multiple randomised controlled trials (RCTs) for the prophylaxis and treatment of cancer-associated venous thromboembolism (VTE) leading to changes in guidelines. To quantify the risks and benefits of DOACs in the prophylaxis and treatment of cancer-associated VTE, we performed a systematic review and meta-analysis of published RCTs.
Methods: A systematic search of PubMed, Cochrane Library and Google Scholar databases for all phase-3 RCTs of DOACs in patients with cancer was conducted. Pooled estimates for the cumulative incidence of VTE, recurrent VTE, major bleeding and clinically relevant non-major bleeding (CRNMB) for each arm and pooled hazard ratio (HR) with 95% confidence intervals (CI) for VTE, recurrent VTE, major bleeding, CRNMB and overall survival were calculated by using random-effect model.
Results: Six phase-3 RCTs (N = 4341) which studied DOACs in prophylaxis or treatment of cancer-associated VTE were included. DOACs significantly reduced the risk of VTE versus placebo in prophylaxis (5% versus 9%, HR 0.51 and 95% CI:0.32-0.82) and the risk of recurrent VTE versus low-molecular-weight heparin in the treatment setting (4% versus 9%, HR 0.58 and 95% CI: 0.40-0.87) although, at a cost of increased risk of major bleeding (HR 1.46 and 95% CI: 1.0-2.12) or CRNMB (HR 1.42 and 95% CI: 1.10-1.81), there was no effect on survival (HR 1.01 and 95% CI: 0.85-1.20).
Conclusion: In this meta-analysis, we found that DOACs not only significantly decreased the risk of VTE or recurrent VTE in patients with cancer but also significantly increased the risk of bleeding and CRNMB, with neither beneficial nor detrimental effects on survival. The quantification of these benefits and risks will assist in individualised shared decision-making.
Keywords: apixaban; direct oral anticoagulants; edoxaban; rivaroxaban; venous thromboembolism.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
None.
Figures
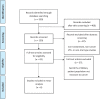











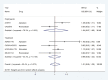
References
-
- Stare J, Maucort-Boulch D. Odds ratio, hazard ratio and relative risk. Metodoloski zvezki. 2017;13:59–67.
LinkOut - more resources
Full Text Sources
