Cost-effectiveness analysis of alternative colon cancer screening strategies in the context of the French national screening program
- PMID: 33014138
- PMCID: PMC7509710
- DOI: 10.1177/1756284820953364
Cost-effectiveness analysis of alternative colon cancer screening strategies in the context of the French national screening program
Abstract
Background: A nationwide colorectal cancer (CRC) screening program was set up in France from 2009 for average-risk, asymptomatic people aged 50-74 years based on an immunochemical fecal occult blood test [faecal immunochemical test (FIT)] every 2 years, followed by colonoscopy if positive. The European standard recommends a participation rate of 45% for the program to be cost-effective, yet the latest published rate in France was 34%. The objective of this study was to compare the cost effectiveness of screening alternatives taking real-world participation rates into account.
Methods: Eight screening strategies were compared, based either on a screening test (Guaiac or FIT testing, blood-based, stool DNA, computed tomography colonography, colon capsules, and sigmoidoscopy) followed by full colonoscopy if positive or direct colonoscopy. A microsimulation model was used to estimate the cost effectiveness associated with each strategy.
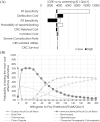
Results: Compared with no screening, FIT was associated with a 14.0 quality-adjusted life year (QALY) increase of €50,520 per 1000 individuals, giving an incremental cost-effectiveness ratio (ICER) of €3600/QALY. Only stool DNA and blood-based testing were associated with a QALY increase compared with FIT, with stool DNA weakly dominated by blood-based testing, and the latter associated with an ICER of €154,600/QALY compared with FIT. All other strategies were dominated by FIT.
Conclusion: FIT every 2 years appears to be the most cost-effective CRC screening strategy when taking into account a real-world participation rate of 34%.
Keywords: FIT; colorectal cancer; cost-effectiveness; screening.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: S Barré, H Leleu, A Vimont, S Taleb, JC Saurin and F De Bels declare no conflict of interest. R Benamouzig has received honoraria from Alfasigm, Bayer, Medtronic and Mayoli.
Figures

References
-
- Leone N, Voirin N, Roche L, et al. Projection de l’incidence et de la mortalité par cancer en France métropolitaine en 2015. Rapport technique. Saint-Maurice (Fra): Institut de veille sanitaire, 2015, p. 62.
-
- Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer 2006; 107: 1128–1141. - PubMed
-
- Morson BC. Evolution of cancer of the colon and rectum. Cancer 1974; 34(Suppl): 845–849. - PubMed
-
- Institut National de la Santé et de le Recherche Médicale. Cancers: pronostics à long terme. Paris: INSERM, 2006.
-
- Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008; 103: 1541–1549. - PubMed
LinkOut - more resources
Full Text Sources

