Clinicopathologic significance of nuclear HER4 and phospho-YAP(S127) in human breast cancers and matching brain metastases
- PMID: 33014146
- PMCID: PMC7517995
- DOI: 10.1177/1758835920946259
Clinicopathologic significance of nuclear HER4 and phospho-YAP(S127) in human breast cancers and matching brain metastases
Abstract
Background: Human epidermal growth factor receptor-4 (HER4) and yes-associated protein-1 (YAP) are candidate therapeutic targets in oncology. YAP's transcriptional coactivation function is modulated by the HER4 intracellular domain (HER4-ICD) in vitro, but the clinical relevance of this has not been established. This study investigated the potential for targeting the HER4-YAP pathway in brain metastatic breast cancer.
Methods: We performed immuno-phenotypic profiling of pathway markers in a consecutive breast cancer series with 25 years of clinical follow up (n = 371), and patient-matched breast and metastatic brain tumours (n = 91; 30 pairs).
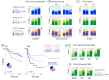
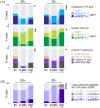
Results: Membrane localisation of phospho-HER4 [pHER4(Y1162)] was infrequent in primary breast cancer, but very frequent in brain metastases (5.9% versus 75% positive), where it was usually co-expressed with pHER3(Y1289) (p < 0.05). The presence of YAP in tumour cell nuclei was associated directly with nuclear pERK5(T218/Y210) (p = 0.003). However, relationships with disease-specific survival depended on oestrogen receptor (ER) status. Nuclear pYAP(S127) was associated with smaller, good prognostic ER+ breast tumours (log-rank hazard-ratio 0.53; p = 9.6E-03), but larger, poor prognostic triple-negative cancers (log-rank hazard-ratio 2.78; p = 1.7E-02), particularly when co-expressed with nuclear HER4-ICD (p = 0.02). This phenotype was associated with stemness and mitotic instability markers (vimentin, SOX9, ID1, SPAG5, TTK, geminin; p < 0.05). YAP expression in brain metastases was higher than matched primary tumours; specifically, nuclear pYAP(S127) in ER-negative cases (p < 0.05). Nuclear YAP was detected in ~70% of ER-negative, HER4-activated brain metastases.
Discussion: Our findings suggest that the canonical-mechanism where Hippo pathway-mediated phosphorylation of YAP ostensibly excludes it from the nucleus is dysfunctional in breast cancer. The data are consistent with pYAP(S127) having independent transcriptional functions, which may include transducing neuregulin signals in brain metastases. Consistent with mechanistic studies implicating it as an ER co-factor, nuclear pYAP(S127) associations with breast cancer clinical outcomes were dependent on ER status.
Conclusion: Preclinical studies investigating HER4 and nuclear YAP combination therapy strategies are warranted.
Keywords: HER4; biomarkers; brain metastasis; breast cancer.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer.Int J Clin Exp Pathol. 2014 May 15;7(6):3224-34. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25031743 Free PMC article.
-
Differential expression of Yes-associated protein and phosphorylated Yes-associated protein is correlated with expression of Ki-67 and phospho-ERK in colorectal adenocarcinoma.Histol Histopathol. 2013 Nov;28(11):1483-90. doi: 10.14670/HH-28.1483. Epub 2013 May 15. Histol Histopathol. 2013. PMID: 23673988
-
Expression of Yes-associated protein (YAP) in metastatic breast cancer.Int J Clin Exp Pathol. 2015 Sep 1;8(9):11248-57. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26617849 Free PMC article.
-
Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues.Hum Pathol. 2017 Oct;68:166-174. doi: 10.1016/j.humpath.2017.08.032. Epub 2017 Sep 9. Hum Pathol. 2017. PMID: 28899737 Review.
-
Dual roles of yes-associated protein (YAP) in colorectal cancer.Oncotarget. 2017 Aug 11;8(43):75727-75741. doi: 10.18632/oncotarget.20155. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088905 Free PMC article. Review.
Cited by
-
Characterization of Immune Cell Subsets of Tumor Infiltrating Lymphocytes in Brain Metastases.Biology (Basel). 2021 May 11;10(5):425. doi: 10.3390/biology10050425. Biology (Basel). 2021. PMID: 34064871 Free PMC article.
-
Emerging Biomarkers for Diagnosis, Prevention and Treatment of Brain Metastases-From Biology to Clinical Utility.Diseases. 2022 Feb 3;10(1):11. doi: 10.3390/diseases10010011. Diseases. 2022. PMID: 35225863 Free PMC article. Review.
-
Landscape of Epidermal Growth Factor Receptor Heterodimers in Brain Metastases.Cancers (Basel). 2022 Jan 21;14(3):533. doi: 10.3390/cancers14030533. Cancers (Basel). 2022. PMID: 35158800 Free PMC article.
-
COMMD3 loss drives invasive breast cancer growth by modulating copper homeostasis.J Exp Clin Cancer Res. 2023 Apr 18;42(1):90. doi: 10.1186/s13046-023-02663-8. J Exp Clin Cancer Res. 2023. PMID: 37072858 Free PMC article.
-
Epigenome erosion and SOX10 drive neural crest phenotypic mimicry in triple-negative breast cancer.NPJ Breast Cancer. 2022 May 2;8(1):57. doi: 10.1038/s41523-022-00425-x. NPJ Breast Cancer. 2022. PMID: 35501337 Free PMC article.
References
-
- Tabouret E, Chinot O, Metellus P, et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res 2012; 32: 4655–4662. - PubMed
-
- Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol 1978; 19: 579–592. - PubMed
-
- Percy AK. Neoplasms of the central nervous system: epidemiologic considerations. Neurology 1970; 20: 398–399. - PubMed
-
- Tsukada Y, Fouad A, Pickren JW, et al. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 1983; 52: 2349–2354. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

