Peritumoral activated hepatic stellate cells are associated with hepatic recurrence for resectable colorectal adenocarcinoma liver metastasis following resection
- PMID: 33014165
- PMCID: PMC7520724
- DOI: 10.3892/ol.2020.12150
Peritumoral activated hepatic stellate cells are associated with hepatic recurrence for resectable colorectal adenocarcinoma liver metastasis following resection
Abstract
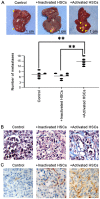
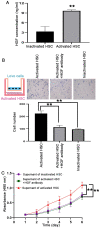
The formation of the pre-metastatic niche (PMN), which precedes the establishment of tumor lesions, plays a critical role in cancer recurrence and metastasis. Hepatic stellate cells (HSCs), a critical liver stromal cell component, can be induced to facilitate metastasis by modeling liver PMN formation. In the present study, activated HSCs were observed in the peritumor non-cancerous liver tissues (PNLT) colorectal adenocarcinoma liver metastasis (CRALM), and the density of activated HSCs was higher in PNLT compared with that in normal liver tissues (NLT). High density of activated HSC in the PNLT was positively associated with the number of tumor liver metastases (P=0.036), maximum diameter of liver metastases (P=0.002), and recurrence following synchronous radical resection (P=0.003). High density of activated HSCs in the PNLT was identified as a significant and independent prognostic factor for disease-free survival (HR, 2.083; 95% CI, 1.504-2.885; P=0.016) and overall survival (HR, 2.039; 95% CI, 1.312-3.169; P=0.019). Functionally, in vitro assays revealed that activated HSCs facilitated colorectal adenocarcinoma (CRA) cells to colonize the liver. Molecularly, it was demonstrated that the pro-recurrence of activated HSCs depended on paracrine hepatic growth factor. Taken together, the present results showed that high density of activated HSCs in the PNLT was an independent predictor for CRALM recurrence following resection, and they exerted their roles via their effect on CRA cell recruitment and proliferation by paracrine HGF.
Keywords: colorectal cancer; hepatic recurrence; hepatic stellate cells; liver metastasis; pre-metastatic niche.
Copyright: © Deng et al.
Figures



Similar articles
-
Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver.Gastroenterology. 2016 Nov;151(5):1011-1024.e7. doi: 10.1053/j.gastro.2016.07.043. Epub 2016 Aug 6. Gastroenterology. 2016. PMID: 27506299
-
Role of Fibroblast Growth Factors in the Crosstalk of Hepatic Stellate Cells and Uveal Melanoma Cells in the Liver Metastatic Niche.Int J Mol Sci. 2022 Sep 29;23(19):11524. doi: 10.3390/ijms231911524. Int J Mol Sci. 2022. PMID: 36232829 Free PMC article.
-
Proof of prometastatic niche induction by hepatic stellate cells.J Surg Res. 2015 Apr;194(2):496-504. doi: 10.1016/j.jss.2014.11.005. Epub 2014 Nov 10. J Surg Res. 2015. PMID: 25528682
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Apoptotic and survival signals in hepatic stellate cells.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007 Oct;32(5):726-34. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2007. PMID: 18007061 Review.
Cited by
-
Exosomal miR-106a-5p from highly metastatic colorectal cancer cells drives liver metastasis by inducing macrophage M2 polarization in the tumor microenvironment.J Exp Clin Cancer Res. 2024 Oct 9;43(1):281. doi: 10.1186/s13046-024-03204-7. J Exp Clin Cancer Res. 2024. PMID: 39385295 Free PMC article.
-
Colorectal cancer cells-derived exosomal miR-188-3p promotes liver metastasis by creating a pre-metastatic niche via activation of hepatic stellate cells.J Transl Med. 2025 Mar 25;23(1):369. doi: 10.1186/s12967-025-06334-4. J Transl Med. 2025. PMID: 40134019 Free PMC article.
-
Depletion of tumor-reactive HSCs reveals their significance during different stages of liver metastasis.Hepatol Commun. 2025 Mar 24;9(4):e0669. doi: 10.1097/HC9.0000000000000669. eCollection 2025 Apr 1. Hepatol Commun. 2025. PMID: 40130990 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Brodt P. Role of the microenvironment in liver metastasis: From Pre- to prometastatic niches. Clin Cancer Res. 2016;22:5971–5982. - PubMed
-
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. - PubMed
LinkOut - more resources
Full Text Sources
