Styryl-based new organic chromophores bearing free amino and azomethine groups: synthesis, photophysical, NLO, and thermal properties
- PMID: 33014168
- PMCID: PMC7509381
- DOI: 10.3762/bjoc.16.189
Styryl-based new organic chromophores bearing free amino and azomethine groups: synthesis, photophysical, NLO, and thermal properties
Abstract
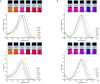
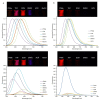
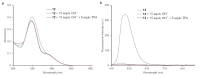
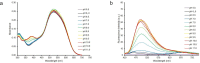
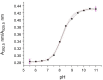
Herein we report the synthesis and characterization of a new series of styryl-based push-pull dyes containing a free amino group and their Schiff base derivatives. The dyes include the dicyanomethylene group as an acceptor and different para-substituted alkylamines as donors. Morever as a proton-sensitive group a pyridin-2-yl substituent was attached to the para-position of the phenyl moiety in both series of compounds. The photophysical properties of the dyes were examined in various solvents with different polarities and showed absorption in the visible region and green-red emission with low quantum yields. The absorption and the emission maxima were shifted bathocromically by increasing the solvent's polarity. However, there was no correlation with the polarity parameters of the solvents. The pH-sensitive properties of all prepared Schiff bases were examined against TBAOH in DMSO, via deprotonation of the OH group in the salicylidene moiety and their reverse protonation was also investigated using TFA. The Schiff bases exhibited a bathochromic shift upon the addition of TBAOH to their solutions in DMSO. Therefore, they showed potential to be utilized as colorimetric and luminescence pH sensors. The second-order nonlinear optical (NLO) responses of the dyes were measured by the electric field-induced second harmonic (EFISH) generation method. The highest μβ values were obtained for the dyes bearing the julolidine donor as 1430 × 10-48 esu (for free amino derivative) and 1950 × 10-48 esu (for Schiff base derivative), respectively. The structural and electronic properties of the dyes as well as their NLO properties were further studied using DFT calculations. The thermal stabilities of all dyes were evaluated by thermogravimetric analysis (TGA). The TGA data showed that all dyes were thermally stable up to 250 °C.
Keywords: DFT calculations; NLO; Schiff base; pH sensitive dyes; solvent effect; styryl dyes.
Copyright © 2020, Putra et al.; licensee Beilstein-Institut.
Figures






References
-
- Valeur B, Berberan-Santos M N. Molecular Fluorescence: Principles and Applications. 2nd ed. Weinheim, Germany: Wiley-VCH; 2012. - DOI
-
- Gore M. Spectrophotometry and Spectrofluorimetry: A Practical Approach. Oxford, U.K.: Oxford University Press; 2000.
-
- Braslavsky S E. Pure Appl Chem. 2007;79:293–465. doi: 10.1351/pac200779030293. - DOI
-
- Williams D J. Angew Chem, Int Ed Engl. 1984;23:690–703. doi: 10.1002/anie.198406901. - DOI
-
- Boyd R W. Nonlineaer Optics. 2nd ed. San Diego, CA, USA: Academic Press; 2003. - DOI
LinkOut - more resources
Full Text Sources
