Impact of Ninjin'Yoeito on Fatigue in Patients Receiving Nab-Paclitaxel Plus Gemcitabine Therapy: A Prospective, Single-Arm, Phase II Open Label, Nonrandomized, Historically-Controlled Study
- PMID: 33014206
- PMCID: PMC7522496
- DOI: 10.1016/j.curtheres.2020.100605
Impact of Ninjin'Yoeito on Fatigue in Patients Receiving Nab-Paclitaxel Plus Gemcitabine Therapy: A Prospective, Single-Arm, Phase II Open Label, Nonrandomized, Historically-Controlled Study
Abstract
Background: Ninjin'yoeito, a traditional Japanese herbal medicine, is used to prevent fatigue, loss of appetite, and coldness of limbs. Fatigue is an especially common issue during chemotherapy and can affect quality of life and the ability to complete scheduled treatment.
Objectives: This prospective exploratory trial evaluates the efficacy of ninjin'yoeito for fatigue in patients undergoing nab-paclitaxel plus gemcitabine therapy for unresectable pancreatic cancer. The primary end point was evaluation of fatigue according to Functional Assessment of Chronic Illness Therapy-Fatigue score during 2 courses of nab-paclitaxel plus gemcitabine therapy. Secondary end points included evaluation of dose intensity, appetite loss using numerical rating scale, and peripheral neuropathy using a patient neurotoxicity questionnaire.
Methods: We compared data from this interventional trial with a prior observational trial without administration of ninjin'yoeito with identical definition of end points (UMIN000021758). Thirty patients were required by the study.
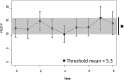
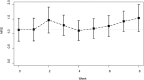
Results: Threshold mean of Functional Assessment of Chronic Illness Therapy-Fatigue score across 8 weeks during chemotherapy was under 5.3 (P = 0.002). Secondary end points did not reveal any specific patterns in appetite loss or degree of pain. No significant changes in patient neurotoxicity questionnaire concerning sensory/motor disorders were observed, but the mean (SD) incidence of patients with sensory disturbance was higher between the fifth and eighth weeks (8.8 [1.26]) than during the first and fourth weeks (4.8 [0.96]) (P = 0.003). Clinically significant adverse reactions of ninjin'yoeito were not observed.
Conclusions: Ninjin'yoeito may be useful for improving the symptoms of fatigue caused by nab-paclitaxel plus gemcitabine in patients with unresectable pancreatic cancer. UMIN Clinical Trials Registry identifier: UMIN000025606. (Curr Ther Res Clin Exp. 2020; 81:XXX-XXX).
Keywords: Fatigue; Japanese herbal medicine; Nab-paclitaxel plus gemcitabine; Ninjin'yoeito; Supportive therapy.
© 2020 The Author(s).
Figures
Similar articles
-
Prospective validation of patient fatigue questionnaire (FACIT-F) for fatigue assessment in nab-paclitaxel plus gemcitabine therapy.Mol Clin Oncol. 2018 Jan;8(1):121-126. doi: 10.3892/mco.2017.1485. Epub 2017 Nov 3. Mol Clin Oncol. 2018. PMID: 29387403 Free PMC article.
-
Combined Use of Ninjin'yoeito Improves Subjective Fatigue Caused by Lenalidomide in Patients With Multiple Myeloma: A Retrospective Study.Front Nutr. 2018 Aug 21;5:72. doi: 10.3389/fnut.2018.00072. eCollection 2018. Front Nutr. 2018. PMID: 30186837 Free PMC article.
-
Safety and Effectiveness of Ninjin'yoeito: A Utilization Study in Elderly Patients.Front Nutr. 2019 Feb 19;6:14. doi: 10.3389/fnut.2019.00014. eCollection 2019. Front Nutr. 2019. PMID: 30873411 Free PMC article.
-
Herbal Medicine Ninjin'yoeito in the Treatment of Sarcopenia and Frailty.Front Nutr. 2018 Dec 12;5:126. doi: 10.3389/fnut.2018.00126. eCollection 2018. Front Nutr. 2018. PMID: 30619872 Free PMC article. Review.
-
Kampo medicines for supportive care of patients with cancer: A brief review.Integr Med Res. 2022 Jun;11(2):100839. doi: 10.1016/j.imr.2022.100839. Epub 2022 Feb 23. Integr Med Res. 2022. PMID: 35242536 Free PMC article. Review.
Cited by
-
Immunological Differences in Human Peripheral Blood Mononuclear Cells Treated with Traditional Japanese Herbal Medicines Hochuekkito, Juzentaihoto, and Ninjin'yoeito from Different Pharmaceutical Companies.Evid Based Complement Alternat Med. 2021 Sep 18;2021:7605057. doi: 10.1155/2021/7605057. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34589133 Free PMC article.
-
Renshen Yangrong decoction for secondary malaise and fatigue: network pharmacology and Mendelian randomization study.Front Nutr. 2024 Jun 20;11:1404123. doi: 10.3389/fnut.2024.1404123. eCollection 2024. Front Nutr. 2024. PMID: 38966421 Free PMC article.
-
Safety and efficacy of Ninjin'yoeito along with iron supplementation therapy for preoperative anemia, fatigue, and anxiety in patients with gynecological disease: an open-label, single-center, randomized phase-II trial.BMC Womens Health. 2022 Jun 14;22(1):229. doi: 10.1186/s12905-022-01824-9. BMC Womens Health. 2022. PMID: 35701778 Free PMC article. Clinical Trial.
References
-
- Rahib L, Smith BD, Aizenberg R. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Conroy T, Desseigne F, Ychou M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. - PubMed
-
- Okada KI, Hirono S, Kawai M. Phase I Study of Nab-Paclitaxel plus Gemcitabine as Neoadjuvant Therapy for Borderline Resectable Pancreatic Cancer. Anticancer Res. 2017;37:853–858. - PubMed
LinkOut - more resources
Full Text Sources

