Monitoring of Lassa virus infection in suspected and confirmed cases in Ondo State, Nigeria
- PMID: 33014249
- PMCID: PMC7519794
- DOI: 10.11604/pamj.2020.36.253.22104
Monitoring of Lassa virus infection in suspected and confirmed cases in Ondo State, Nigeria
Abstract
Introduction: Lassa virus (LASV), the causative agent of Lassa fever (LF), an endemic acute viral haemorrhagic illness in Nigeria, is transmitted by direct contact with the rodent, contaminated food or household items. Person-to-person transmission also occurs and sexual transmission has been reported. Thus, this study investigated the presence of LASV in body fluids of suspected and confirmed cases.
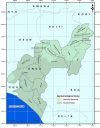
Methods: this was a cross-sectional study between March 2018 and April 2019 involving 112 consenting suspected and post ribavirin confirmed cases attending the Lassa fever treatment center in Ondo State. Whole blood was collected from 57 suspected and 29 confirmed cases. Other samples from confirmed cases were 5 each of High Vaginal Swab (HVS) and seminal fluid; 12 breast milk and 4 urine. All samples were analyzed using reverse transcription-PCR (RT-PCR) targeting the S-gene of LASV.
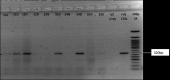
Results: analysis of whole blood by RT-PCR showed that 1/57 (1.8%) suspected and 1/29 (3.4%) confirmed post ribavirin treated cases were positive. While LASV was detected in 2/5 (40%) post ribavirin treated seminal fluids and 1/11 (8.3%) breast milk. However, LASV was not detected in any of the HVS and urine samples.
Conclusion: the detection of LASV in seminal fluid and breast milk of discharged post ribavirin treated cases suggests its persistence in these fluids of recovering Nigerians. The role of postnatal and sexual transmissions in the perennial outbreak of LF needs to be further evaluated.
Keywords: Lassa Virus; Lassa fever; Ondo; reverse transcription polymerase chain reaction; transmission.
Copyright: Olumuyiwa Babalola Salu et al.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Lassa virus RNA detection from suspected cases in Nigeria, 2011-2017.Pan Afr Med J. 2019 Oct 6;34:76. doi: 10.11604/pamj.2019.34.76.16425. eCollection 2019. Pan Afr Med J. 2019. PMID: 31819792 Free PMC article.
-
Increased Prevalence of Lassa Fever Virus-Positive Rodents and Diversity of Infected Species Found during Human Lassa Fever Epidemics in Nigeria.Microbiol Spectr. 2022 Aug 31;10(4):e0036622. doi: 10.1128/spectrum.00366-22. Epub 2022 Aug 1. Microbiol Spectr. 2022. PMID: 35913205 Free PMC article.
-
Lassa fever in post-conflict sierra leone.PLoS Negl Trop Dis. 2014 Mar 20;8(3):e2748. doi: 10.1371/journal.pntd.0002748. eCollection 2014 Mar. PLoS Negl Trop Dis. 2014. PMID: 24651047 Free PMC article.
-
Current research for a vaccine against Lassa hemorrhagic fever virus.Drug Des Devel Ther. 2018 Aug 14;12:2519-2527. doi: 10.2147/DDDT.S147276. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30147299 Free PMC article. Review.
-
Vaccine platforms for the prevention of Lassa fever.Immunol Lett. 2019 Nov;215:1-11. doi: 10.1016/j.imlet.2019.03.008. Epub 2019 Apr 23. Immunol Lett. 2019. PMID: 31026485 Free PMC article. Review.
Cited by
-
Treatment of highly virulent mammarenavirus infections-status quo and future directions.Expert Opin Drug Discov. 2024 May;19(5):537-551. doi: 10.1080/17460441.2024.2340494. Epub 2024 Apr 12. Expert Opin Drug Discov. 2024. PMID: 38606475 Free PMC article. Review.
-
Genomic changes of Lassa virus associated with mammalian host adaptation.BMC Genomics. 2025 May 15;26(1):489. doi: 10.1186/s12864-025-11666-y. BMC Genomics. 2025. PMID: 40375146 Free PMC article.
-
Purification and characterization of the Lassa virus transmembrane domain.Biochem Biophys Rep. 2022 Dec 18;33:101409. doi: 10.1016/j.bbrep.2022.101409. eCollection 2023 Mar. Biochem Biophys Rep. 2022. PMID: 36583076 Free PMC article.
-
Viral RNA and infectious virus in mucosal specimens from guinea pigs modelling early phases of lethal and non-lethal Lassa fever.Emerg Microbes Infect. 2022 Dec;11(1):1390-1393. doi: 10.1080/22221751.2022.2071637. Emerg Microbes Infect. 2022. PMID: 35481464 Free PMC article.
-
Correlation between viral infections in male semen and infertility: a literature review.Virol J. 2024 Jul 30;21(1):167. doi: 10.1186/s12985-024-02431-w. Virol J. 2024. PMID: 39080728 Free PMC article. Review.
References
-
- Enria DA, Mills JN, Bausch D, Shieh W, Peters CJ. Arenavirus infections. Tropical Infectious Diseases: Principles, Pathogens, and Practice, Elsevier, Philadelphia, PA. 2011;3:449–461.
-
- CDC. Imported Lassa fever--New Jersey, 2004. MMWR Morb Mortal Wkly Rep. 2004 Oct 1;53(38):894–897. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
