COVID-19: Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm
- PMID: 33014275
- PMCID: PMC7519203
- DOI: 10.1155/2020/6401341
COVID-19: Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm
Abstract
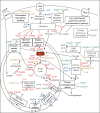
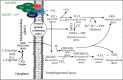
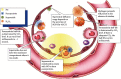
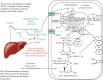
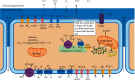
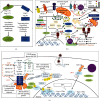
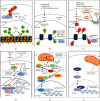
Human SARS-CoV-2 infection is characterized by a high mortality rate due to some patients developing a large innate immune response associated with a cytokine storm and acute respiratory distress syndrome (ARDS). This is characterized at the molecular level by decreased energy metabolism, altered redox state, oxidative damage, and cell death. Therapies that increase levels of (R)-beta-hydroxybutyrate (R-BHB), such as the ketogenic diet or consuming exogenous ketones, should restore altered energy metabolism and redox state. R-BHB activates anti-inflammatory GPR109A signaling and inhibits the NLRP3 inflammasome and histone deacetylases, while a ketogenic diet has been shown to protect mice from influenza virus infection through a protective γδ T cell response and by increasing electron transport chain gene expression to restore energy metabolism. During a virus-induced cytokine storm, metabolic flexibility is compromised due to increased levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that damage, downregulate, or inactivate many enzymes of central metabolism including the pyruvate dehydrogenase complex (PDC). This leads to an energy and redox crisis that decreases B and T cell proliferation and results in increased cytokine production and cell death. It is hypothesized that a moderately high-fat diet together with exogenous ketone supplementation at the first signs of respiratory distress will increase mitochondrial metabolism by bypassing the block at PDC. R-BHB-mediated restoration of nucleotide coenzyme ratios and redox state should decrease ROS and RNS to blunt the innate immune response and the associated cytokine storm, allowing the proliferation of cells responsible for adaptive immunity. Limitations of the proposed therapy include the following: it is unknown if human immune and lung cell functions are enhanced by ketosis, the risk of ketoacidosis must be assessed prior to initiating treatment, and permissive dietary fat and carbohydrate levels for exogenous ketones to boost immune function are not yet established. The third limitation could be addressed by studies with influenza-infected mice. A clinical study is warranted where COVID-19 patients consume a permissive diet combined with ketone ester to raise blood ketone levels to 1 to 2 mM with measured outcomes of symptom severity, length of infection, and case fatality rate.
Copyright © 2020 Patrick C. Bradshaw et al.
Conflict of interest statement
The authors declare no competing interests. Dr. William Seeds has no current role in the operation and no financial interest in drseeds.com, and his legal separation from that entity is pending.
Figures


Similar articles
-
Impaired ketogenesis ties metabolism to T cell dysfunction in COVID-19.Nature. 2022 Sep;609(7928):801-807. doi: 10.1038/s41586-022-05128-8. Epub 2022 Jul 28. Nature. 2022. PMID: 35901960 Free PMC article.
-
Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19.J Biol Regul Homeost Agents. 2020 Sep-Oct,;34(5):1629-1632. doi: 10.23812/20-2EDIT. J Biol Regul Homeost Agents. 2020. PMID: 32945158
-
Targeting the NLRP3 Inflammasome in Severe COVID-19.Front Immunol. 2020 Jun 23;11:1518. doi: 10.3389/fimmu.2020.01518. eCollection 2020. Front Immunol. 2020. PMID: 32655582 Free PMC article. Review.
-
The dark side of the spoon - glucose, ketones and COVID-19: a possible role for ketogenic diet?J Transl Med. 2020 Nov 20;18(1):441. doi: 10.1186/s12967-020-02600-9. J Transl Med. 2020. PMID: 33218357 Free PMC article. Review.
-
A combinatorial approach of a polypharmacological adjuvant 2-deoxy-D-glucose with low dose radiation therapy to quell the cytokine storm in COVID-19 management.Int J Radiat Biol. 2020 Nov;96(11):1323-1328. doi: 10.1080/09553002.2020.1818865. Epub 2020 Oct 6. Int J Radiat Biol. 2020. PMID: 32910699
Cited by
-
Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations.Signal Transduct Target Ther. 2022 Jan 17;7(1):11. doi: 10.1038/s41392-021-00831-w. Signal Transduct Target Ther. 2022. PMID: 35034957 Free PMC article. Review.
-
Intermittent hypoxia: linkage between OSAS and epilepsy.Front Pharmacol. 2023 Nov 23;14:1230313. doi: 10.3389/fphar.2023.1230313. eCollection 2023. Front Pharmacol. 2023. PMID: 38074156 Free PMC article. Review.
-
Role of senescence in the chronic health consequences of COVID-19.Transl Res. 2022 Mar;241:96-108. doi: 10.1016/j.trsl.2021.10.003. Epub 2021 Oct 22. Transl Res. 2022. PMID: 34695606 Free PMC article. Review.
-
Pulmonary redox imbalance drives early fibroproliferative response in moderate/severe coronavirus disease-19 acute respiratory distress syndrome and impacts long-term lung abnormalities.Ann Intensive Care. 2024 May 12;14(1):72. doi: 10.1186/s13613-024-01293-3. Ann Intensive Care. 2024. PMID: 38735020 Free PMC article.
-
Inflammatory Bowel Disease and COVID-19: How Microbiomics and Metabolomics Depict Two Sides of the Same Coin.Front Microbiol. 2022 Mar 21;13:856165. doi: 10.3389/fmicb.2022.856165. eCollection 2022. Front Microbiol. 2022. PMID: 35391730 Free PMC article. Review.
References
-
- Islam M. R., Fischer A. A Transcriptome Analysis Identifies Potential Preventive and Therapeutic Approaches Towards COVID-19. Preprints; 2020. - DOI
-
- Kamepalli R., Kamepalli B. How immune T-cell augmentation can help prevent COVID-19: a possible nutritional solution using ketogenic lifestyle. The University of Louisville Journal of Respiratory Infections. 2020;4:7.
-
- Hamming I., Timens W., Bulthuis M. L. C., Lely A. T., Navis G. J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology. 2004;203(2):631–637. doi: 10.1002/path.1570. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

