Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness
- PMID: 33014286
- PMCID: PMC7517961
- DOI: 10.18632/oncotarget.27748
Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness
Abstract
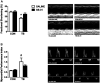
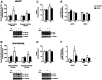
Cancer cachexia is a syndrome characterized by profound cardiac and diaphragm muscle wasting, which increase the risk of morbidity in cancer patients due to failure of the cardiorespiratory system. In this regard, muscle relies greatly on mitochondria to meet energy requirements for contraction and mitochondrial dysfunction can result in muscle weakness and fatigue. In addition, mitochondria are a major source of reactive oxygen species (ROS) production, which can stimulate increased rates of muscle protein degradation. Therefore, it has been suggested that mitochondrial dysfunction may be an underlying factor that contributes to the pathology of cancer cachexia. To determine if pharmacologically targeting mitochondrial dysfunction via treatment with the mitochondria-targeting peptide SS-31 would prevent cardiorespiratory muscle dysfunction, colon 26 (C26) adenocarcinoma tumor-bearing mice were administered either saline or SS-31 daily (3 mg/kg/day) following inoculation. C26 mice treated with saline demonstrated greater ROS production and mitochondrial uncoupling compared to C26 mice receiving SS-31 in both the heart and diaphragm muscle. In addition, saline-treated C26 mice exhibited a decline in left ventricular function which was significantly rescued in C26 mice treated with SS-31. In the diaphragm, muscle fiber cross-sectional area of C26 mice treated with saline was significantly reduced and force production was impaired compared to C26, SS-31-treated animals. Finally, ventilatory deficits were also attenuated in C26 mice treated with SS-31, compared to saline treatment. These data demonstrate that C26 tumors promote severe cardiac and respiratory myopathy, and that prevention of mitochondrial dysfunction is sufficient to preclude cancer cachexia-induced cardiorespiratory dysfunction.
Keywords: SS-31; cachexia; diaphragm; elamipretide; heart.
Conflict of interest statement
CONFLICTS OF INTEREST Dr. Szeto is the inventor of SS-31 and Founder of Stealth BioTherapeutics.
Figures

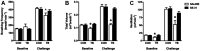

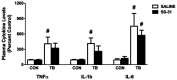
References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, et al.. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011; 12:489–495. 10.1016/S1470-2045(10)70218-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

