Advances in epidermal growth factor receptor specific immunotherapy: lessons to be learned from armed antibodies
- PMID: 33014289
- PMCID: PMC7517958
- DOI: 10.18632/oncotarget.27730
Advances in epidermal growth factor receptor specific immunotherapy: lessons to be learned from armed antibodies
Abstract
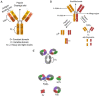
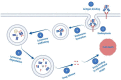
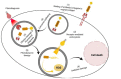
The epidermal growth factor receptor (EGFR) has been recognized as an important therapeutic target in oncology. It is commonly overexpressed in a variety of solid tumors and is critically involved in cell survival, proliferation, metastasis, and angiogenesis. This multi-dimensional role of EGFR in the progression and aggressiveness of cancer, has evolved from conventional to more targeted therapeutic approaches. With the advent of hybridoma technology and phage display techniques, the first anti-EGFR monoclonal antibodies (mAbs) (Cetuximab and Panitumumab) were developed. Due to major limitations including host immune reactions and poor tumor penetration, these antibodies were modified and used as guiding mechanisms for the specific delivery of readily available chemotherapeutic agents or plants/bacterial toxins, giving rise to antibody-drug conjugates (ADCs) and immunotoxins (ITs), respectively. Continued refinement of ITs led to deimmunization strategies based on depletion of B and T-cell epitopes or substitution of non-human toxins leading to a growing repertoire of human enzymes capable of inducing cell death. Similarly, the modification of classical ADCs has resulted in the first, fully recombinant versions. In this review, we discuss significant advancements in EGFR-targeting immunoconjugates, including ITs and recombinant photoactivable ADCs, which serve as a blueprint for further developments in the evolving domain of cancer immunotherapy.
Keywords: epidermal growth factor receptor (EGFR); recombinant antibody photoimmunoconjugates (rAPCs); recombinant antibody-drug conjugates (rADCs); recombinant immunotoxins (ITs); targeted human cytolytic fusion proteins (hCFPs).
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest. Prof Barth is inventor on several patent applications describing EGFR specific PDT and hCFP. Most of these patents have been assigned to UCT.
Figures

References
-
- Dawson JP, Berger MB, Lin C, Schlessinger J, Lemmon MA, Kathryn M, Ferguson KM. Epidermal Growth Factor Receptor Dimerization and Activation Require Ligand-Induced Conformational Changes in the Dimer Interface Epidermal Growth Factor Receptor Dimerization and Activation Require Ligand-Induced Conformational Changes in the Dimer Inter. Mol Cell Biol. 2005; 25:7734–7742. 10.1128/MCB.25.17.7734-7742.2005. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

