Incidence of venous thrombosis after peg-asparaginase in adolescent and young adults with acute lymphoblastic leukemia
- PMID: 33014332
- PMCID: PMC7521187
- DOI: 10.2217/ijh-2020-0009
Incidence of venous thrombosis after peg-asparaginase in adolescent and young adults with acute lymphoblastic leukemia
Abstract
Aim: There are limited data describing incidence of symptomatic venous thromboembolism (VTE) in adolescent and young adult (AYA) acute lymphoblastic leukemia (ALL) patients receiving peg-asparaginase.
Materials & methods: Single-institution retrospective analysis of 44 AYA ALL patients treated with peg-asparaginase. Rates of VTE and proposed risk factors were assessed.
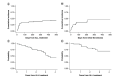
Results: 18 patients (41%) had a symptomatic VTE following peg-asparaginase. The cumulative incidence rate was 25% (95% CI: 13-38%) within 30 days of the initial dose. Personal history of thrombosis was statistically significantly associated with an increased risk of VTE with HR of 2.73 (95% CI: 1.40-5.33, p = 0.003) after adjusting for gender.
Conclusion: These data indicate a high rate of VTE in the AYA ALL population following treatment with peg-asparaginase.
Keywords: acute lymphoblastic leukemia; adolescent young adult; peg-asparaginase; risk factors; venous thromboembolism.
© 2020 Bhavana Bhatnagar.
Conflict of interest statement
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- Amylon MD, Shuster J, Pullen J. et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia 13(3), 335–342 (1999). - PubMed
-
- Pession A, Valsecchi MG, Masera G. et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J. Clin. Oncol. 23(28), 7161–7167 (2005). - PubMed
-
- Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 7(9), 600–606 (2009). - PubMed
-
- Wetzler M, Sanford BL, Kurtzberg J. et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood 109(10), 4164–4167 (2007). - PMC - PubMed
-
• Peg-asparaginase was demonstrated to improve outcomes when used in adult acute lymphoblastic leukemia (ALL) regimens.
-
- Kantarjian H, Thomas D, O'brien S. et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer 101(12), 2788–2801 (2004). - PubMed
LinkOut - more resources
Full Text Sources
