A Single Centre Randomised Control Study to Assess the Impact of Pre-Operative Carbohydrate Loading on Women Undergoing Major Surgery for Epithelial Ovarian Cancer
- PMID: 33014663
- PMCID: PMC7526975
- DOI: 10.7759/cureus.10169
A Single Centre Randomised Control Study to Assess the Impact of Pre-Operative Carbohydrate Loading on Women Undergoing Major Surgery for Epithelial Ovarian Cancer
Abstract
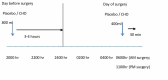
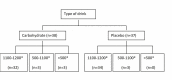
Objective Historically, patients have fasted before elective surgery to ensure an empty stomach to avoid aspiration. A fasting-induced catabolic state however may adversely influence recovery after surgery. Our study was designed to test the effect of oral carbohydrate loading on clinical parameters in patients undergoing major surgery for advanced-stage ovarian cancer. Methods A double-blinded single-centre randomised trial was designed to recruit 110 patients with advanced-stage epithelial ovarian cancer undergoing either primary surgery, or neoadjuvant chemotherapy prior to debulking surgery. Following written informed consent, the patients were randomised into two groups. Group 1 received the carbohydrate drink (intervention) and group 2 received flavoured water (placebo). The quantity of fluid in both groups was 800ml the night before the surgery and 400ml two hours before the induction of anaesthesia. The primary endpoint of the study was the Length of Hospital Stay (LoHS); the secondary parameters assessed were pain scores, nausea and vomiting scores, bowel function, and postoperative complication rate. Results Between March 2009 and December 2011, 80 patients were randomised and 75 completed the study. A decision was made to close the trial early as a change in routine clinical practice meant that patients were admitted on the day of surgery rather than a day before. Analysis of the data revealed that there were no significant differences between the study groups in terms of LoHS and other clinical parameters. Conclusion In this single-center study, which failed to recruit the planned number of patients, we were unable to demonstrate that oral carbohydrate intake pre-operatively has significant impact on the recovery process or the length of hospitalisation postoperatively. Future studies should examine all aspects of an Enhanced Recovery Program after Surgery as a package as compared to a single element to enhance patient outcome.
Keywords: enhanced recovery after surgery; length of hospital stay; nausea and vomiting scores; oral carbohydrate loading; ovarian cancer; pain scores; post operative care.
Copyright © 2020, Al-Hirmizy et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
LinkOut - more resources
Full Text Sources
