The MOMENTUM Study: An International Registry for the Evidence-Based Introduction of MR-Guided Adaptive Therapy
- PMID: 33014774
- PMCID: PMC7505056
- DOI: 10.3389/fonc.2020.01328
The MOMENTUM Study: An International Registry for the Evidence-Based Introduction of MR-Guided Adaptive Therapy
Abstract
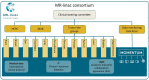
Purpose: MR-guided Radiation Therapy (MRgRT) allows for high-precision radiotherapy under real-time MR visualization. This enables margin reduction and subsequent dose escalation which may lead to higher tumor control and less toxicity. The Unity MR-linac (Elekta AB, Stockholm, Sweden) integrates a linear accelerator with a 1.5T diagnostic quality MRI and an online adaptive workflow. A prospective international registry was established to facilitate the evidence-based implementation of the Unity MR-linac into clinical practice, to systemically evaluate long-term outcomes, and to aid further technical development of MR-linac-based MRgRT. Methods and Results: In February 2019, the Multi-OutcoMe EvaluatioN of radiation Therapy Using the MR-linac study (MOMENTUM) started within the MR-linac Consortium. The MOMENTUM study is an international academic-industrial partnership between several hospitals and industry partner Elekta. All patients treated on the MR-linac are eligible for inclusion in MOMENTUM. For participants, we collect clinical patient data (e.g., patient, tumor, and treatment characteristics) and technical patient data which is defined as information generated on the MR-linac during treatment. The data are captured, pseudonymized, and stored in an international registry at set time intervals up to two years after treatment. Patients can choose to provide patient-reported outcomes and consent to additional MRI scans acquired on the MR-linac. This registry will serve as a data platform that supports multicenter research investigating the MR-linac. Rules and regulations on data sharing, data access, and intellectual property rights are summarized in an academic-industrial collaboration agreement. Data access rules ensure secure data handling and research integrity for investigators and institutions. Separate data access rules exist for academic and industry partners. This study is registered at ClinicalTrials.gov with ID: NCT04075305 (https://clinicaltrials.gov/ct2/show/NCT04075305). Conclusion: The multi-institutional MOMENTUM study has been set up to collect clinical and technical patient data to advance technical development, and facilitate evidenced-based implementation of MR-linac technology with the ultimate purpose to improve tumor control, survival, and quality of life of patients with cancer.
Keywords: MR-guided radiation therapy (MRgRT); MR-linac; MRI; adaptive radiotherapy; functional imaging; image-guidance; magnetic resonance imaging; radiotherapy.
Copyright © 2020 de Mol van Otterloo, Christodouleas, Blezer, Akhiat, Brown, Choudhury, Eggert, Erickson, Faivre-Finn, Fuller, Goldwein, Hafeez, Hall, Harrington, van der Heide, Huddart, Intven, Kirby, Lalondrelle, McCann, Minsky, Mook, Nowee, Oelfke, Orrling, Sahgal, Sarmiento, Schultz, Tersteeg, Tijssen, Tree, van Triest, Hall and Verkooijen.
Figures

References
-
- Winkel D, Kroon PS, Werensteijn-Honingh AM, Bol GH, Raaymakers BW, Jürgenliemk-Schulz IM. Simulated dosimetric impact of online replanning for stereotactic body radiation therapy of lymph node oligometastases on the 1.5T MR-linac. Acta Oncol. (2018) 57:1705–12. 10.1080/0284186X.2018.1512152 - DOI - PubMed
-
- Bainbridge HE, Menten MJ, Fast MF, Nill S, Oelfke U, McDonald F. Treating locally advanced lung cancer with a 1.5 T MR-linac - effects of the magnetic field and irradiation geometry on conventionally fractionated and isotoxic dose-escalated radiotherapy. Radiother Oncol. (2017) 125:280–5. 10.1016/j.radonc.2017.09.009 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

