Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells
- PMID: 33014789
- PMCID: PMC7507989
- DOI: 10.3389/fonc.2020.01483
Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells
Abstract
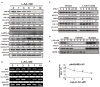
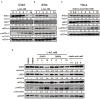
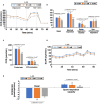
In recent years, L-ascorbic acid (L-AA), or vitamin C, has been attracting attention as a potential anticancer drug that mediates hydrogen peroxide-induced oxidation and ten-eleven translocation 2-catalyzed DNA demethylation. However, the precise mechanism by which L-AA acts remains unclear. We examined the cytotoxic effects of L-AA or sodium ascorbate in human cervical carcinoma cells by assessing cell viability, expression of cell cycle-related mRNAs and proteins, and mitochondrial functions, and by performing flow cytometric analyses of cell cycle profiles, apoptosis, cell proliferation, and production of reactive oxygen species (ROS). We later tested the effects of ascorbates in combination with two first-line chemotherapeutic drugs, cisplatin, and doxorubicin. At pharmacological concentrations (1-10 mM), L-AA increased ROS levels; decreased levels of several cell cycle-related proteins, including p53, p21, cyclin D1, and phosphorylated histone 3 at serine residue 10; induced DNA damage, as indicated by changes in γH2A.x; decreased levels of the anti-oxidative transcription factor Nrf2; and increased levels of catalase, superoxide dismutase 1, and endoplasmic reticulum stress-related indicators, such as the p-eIF2α/eIF2α ratio and CHOP levels. L-AA also promoted cell proliferation and induced apoptosis and mitochondrial dysfunction. Finally, L-AA increased the susceptibility of HeLa cells to cisplatin and doxorubicin. These findings provide insight into how the adjustment of the cellular ROS status through L-ascorbate (L-AA or sodium ascorbate) administration could potentially synergistically enhance the efficacy of cancer therapies.
Keywords: ER stress; L-ascorbic acid; Nrf2; p53; reactive oxygen species.
Copyright © 2020 Wu, Liu, Chen, Chen, Wu and Huang.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

