One-Year Outcome of Multiple Blood-Brain Barrier Disruptions With Temozolomide for the Treatment of Glioblastoma
- PMID: 33014832
- PMCID: PMC7511634
- DOI: 10.3389/fonc.2020.01663
One-Year Outcome of Multiple Blood-Brain Barrier Disruptions With Temozolomide for the Treatment of Glioblastoma
Abstract
Introduction: To overcome the blood-brain barrier (BBB) which interferes with the effect of chemotherapeutic agents, we performed multiple disruptions of BBB (BBBD) with magnetic resonance-guided focused ultrasound on patients with glioblastoma (GBM) during standard adjuvant temozolomide (TMZ) chemotherapy [clinical trial registration no.NCT03712293 (clinicaltrials.gov)]. We report a 1-year follow-up result of BBBD with TMZ for GBM. Methods: From September 2018 to January 2019, six patients were enrolled (four men and two women, median age: 53 years, range: 50-67 years). Of the six patients, five underwent a total of six cycles of BBBD during standard TMZ adjuvant therapy. One patient underwent three cycles of BBBD but continued with TMZ chemotherapy. The 1-year follow-up results of these six patients were reviewed. Results: The mean follow-up duration was 15.17 ± 1.72 months. Two patients showed a recurrence of tumor at 11 and 16 months, respectively. One underwent surgery, and the other patient was restarted with TMZ chemotherapy due to the tumor location with a highly possibility of surgical complications. The survival rate up to 1 year was 100%, and the other four patients are on observation without recurrence. None of the six patients had immediate or delayed BBBD-related complications. Conclusion: Multiple BBBDs can be regarded as a safe procedure without long-term complications, and it seems to have some survival benefits. However, since TMZ partially crosses the BBB, a further extended study with large numbers would be needed to evaluate the benefits of BBBD resulting in an increase of TMZ concentration. This study opened a new therapeutic strategy for GBM by combining BBBD with a larger molecular agent.
Keywords: blood–brain barrier; focused ultrasound; glioblastoma multiforme; magnetic resonance images; progression-free survival.
Copyright © 2020 Park, Kim, Jung, Chang, Choi, Rachmilevitch, Zadicario and Chang.
Figures
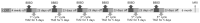
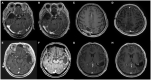

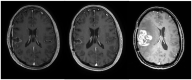
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

