Immune-Related Adverse Events and Corticosteroid Use for Cancer-Related Symptoms Are Associated With Efficacy in Patients With Non-small Cell Lung Cancer Receiving Anti-PD-(L)1 Blockade Agents
- PMID: 33014837
- PMCID: PMC7505083
- DOI: 10.3389/fonc.2020.01677
Immune-Related Adverse Events and Corticosteroid Use for Cancer-Related Symptoms Are Associated With Efficacy in Patients With Non-small Cell Lung Cancer Receiving Anti-PD-(L)1 Blockade Agents
Abstract
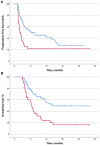
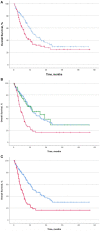
Background: Immune-related adverse events (irAEs) have been associated with improved efficacy in advanced non-small cell lung cancer (NSCLC) patients receiving anti-PD-(L)1 blockade agents, while the concurrent use of corticosteroids seems to worsen it. We evaluated outcomes in advanced NSCLC patients treated with anti-PD-(L)1 blockade agents in relation to the presence of irAEs and the reasons for using corticosteroids: whether for palliative cancer-related reasons or for the management of irAEs. Methods: Clinical outcomes in advanced NSCLC patients treated with anti-PD-(L)1 blockade agents were calculated with regard to the presence of irAEs and the use of corticosteroids. A landmark analysis was performed to avoid immortal time bias due to the time-dependent nature of irAEs. Results: Out of a total of 267 patients, the 56.9% of patients who experienced irAEs had significantly improved outcomes. In the landmark analysis, median progression-free survival (PFS) was 12.4 months for patients with irAEs vs. 4.1 months for patients without irAEs (p < 0.001), while median overall survival (OS) was 28.2 vs. 12.5 months, respectively (p < 0.001). Likewise, objective response and disease control rates were significantly higher in patients experiencing irAEs: 48.6 vs. 22.8% and 77.1 vs. 39.6% (p < 0.001), respectively. Median OS was significantly shorter for patients receiving ≥10 mg of prednisone equivalent daily for cancer-related symptoms than for the rest of patients (<10 mg prednisone equivalent daily or for management of irAEs): 6 vs. 15.9 months (p < 0.001). Conclusions: IrAEs were associated with improved efficacy in advanced NSCLC patients when a landmark analysis was applied. Patients receiving corticosteroids had significantly poorer outcomes when they were used for cancer-related symptoms.
Keywords: advanced NSCLC; corticosteroids; efficacy; immune-related adverse events; immunotherapy.
Copyright © 2020 Riudavets, Mosquera, Garcia-Campelo, Serra, Anguera, Gallardo, Sullivan, Barba, del Carpio, Barnadas, Gich and Majem.
Figures
References
LinkOut - more resources
Full Text Sources
Medical

