Serum SARS-COV-2 Nucleocapsid Protein: A Sensitivity and Specificity Early Diagnostic Marker for SARS-COV-2 Infection
- PMID: 33014893
- PMCID: PMC7498565
- DOI: 10.3389/fcimb.2020.00470
Serum SARS-COV-2 Nucleocapsid Protein: A Sensitivity and Specificity Early Diagnostic Marker for SARS-COV-2 Infection
Abstract
Objective: To explore the diagnostic value of serum severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid (N) protein assay in the early stages of SARS-COV-2 infection. Methods: Serum N protein level in SARS-COV-2 infected patients and non-SARS-COV-2 infected population was measured by enzyme-linked immunosorbent assay (ELISA) double antibody sandwich assay. Colloidal gold immunochromatography assay was used to detect serum N protein antibodies in the above populations. Results: Fifty cases of SARS-CoV-2 nucleic acid-positive and SARS-CoV-2 antibody-negative patients had a serum N protein positivity rate of 76%. Thirty-seven patients who were positive for serum SARS-CoV-2 antibody after infection had a serum SARS-CoV-2 N protein positivity rate of 2.7%. Serum N protein test results of 633 non-SARS-COV-2 infected patients, including pregnant women, patients with other respiratory infections, and individuals with increased rheumatoid factor were all negative, with serum N protein concentration <10.00 pg/mL at 100% specificity. Using SPSS 19.0 to calculate the receiver operating characteristic curve, the area under the curve was determined to be 0.9756 (95% confidence interval 0.9485-1.000, p < 0.0001), and sensitivity and specificity were 92% (95% confidence interval 81.16-96.85%) and 96.84% (95% confidence interval 95.17-97.15%), respectively. The best CUT-OFF value was 1.850 pg/mL. Conclusion: The measurement of serum SARS-COV-2 N protein has a high diagnostic value for infected patients before the antibody appears and shortens the window period of serological diagnosis. It is recommended that the manufacturer establish two different CUT-OFF values according to the purpose of the application. One CUT-OFF value is used for the diagnosis of clinical SARS-COV-2 infection, and the other is used to screen out as many suspected cases as possible.
Keywords: COVID-19; SARS-CoV-2; colloidal gold immunochromatography; diagnostic value; nucleocapsid protein.
Copyright © 2020 Li, Wang, Wang, Li, Zhang, Xu and Wei.
Figures
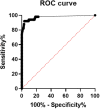

References
-
- Chan J. F., Yip C. C., To K. K., Tang T. H., Wong S. C., Leung K. H., et al. . (2020). Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 58:e00310-20. 10.1128/JCM.00310-20 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

