Comparison of Commercial ELISA Kits to Confirm the Absence of Transmission in Malaria Elimination Settings
- PMID: 33014975
- PMCID: PMC7509087
- DOI: 10.3389/fpubh.2020.00480
Comparison of Commercial ELISA Kits to Confirm the Absence of Transmission in Malaria Elimination Settings
Abstract
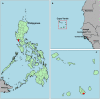
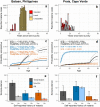
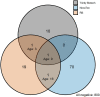
Background: Antimalarial antibody measurements are useful because they reflect historical and recent exposure to malaria. As such, they may provide additional information to assess ongoing transmission in low endemic or pre-elimination settings where cases are rare. In addition, the absence of antibody responses in certain individuals can indicate the cessation of transmission. Commercial malaria enzyme-linked immunosorbent assays (ELISA) detect antimalarial antibodies and are commonly used to screen blood donations for possible malaria infection. However, there is no standardized test to detect antimalarial antibodies for epidemiological use. Here we compared five commercially available ELISA kits (Trinity Biotech, newbio, DiaPro, Cellabs, and NovaTec) in search of a standardized tool for supporting claims of absence of malaria transmission. For comparison, a research-based (RB) ELISA protocol was performed alongside the commercial kits. Results: The commercial kits were first compared using serum samples from known malaria-unexposed individuals (n = 223) and Toxoplasma-infected individuals (n = 191) to assess specificity and cross-reactivity against non-malaria infections. In addition, 134 samples from ≥10-year-olds collected in a hyperendemic region in the Gambia in the early 1990s were used to assess sensitivity. Three out of five kits showed high sensitivity (90-92%), high specificity (98-99%), low cross-reactivity (0-3%) and were considered user-friendly (Trinity Biotech, newbio and NovaTec). Two of these kits (Trinity Biotech and NovaTec) were taken forward for epidemiological evaluation and results were compared to those using the RB-ELISA. Samples from two pre-elimination settings (Praia, Cape Verde; n = 1,396, and Bataan, the Philippines; n = 1,824) were tested. Serological results from both the Trinity Biotech kit and the RB-ELISA concurred with recent passively detected case counts in both settings. Results from the Trinity Biotech kit reflected a significant decrease in the number of reported cases in Bataan in the 1990s better than the RB-ELISA. Results from the NovaTec kit did not reflect transmission patterns in either setting. Conclusion: The Trinity Biotech commercial ELISA kit was considered reliable for epidemiological use and accurately described transmission patterns in two (previously) malaria endemic settings. The use of this simple and standardized serological tool may aid national control and elimination programs by confirming that regions are free from malaria.
Keywords: ELISA; IgG; antibody; commercial ELISA kits; elimination; immunoglobulin; malaria; pre-elimination.
Copyright © 2020 van den Hoogen, Bareng, Alves, Reyes, Macalinao, Rodrigues, Fernandes, Goméz, Hall, Singh, Fornace, Luchavez, Kitchen, Chiodini, Espino, Tetteh, Stresman, Sepúlveda and Drakeley.
Figures
References
-
- Bruce-Chwatt LJ, Draper CC, Avramidis D, Kazandzoglou O. Sero-epidemiological surveillance of disappearing malaria in Greece. J Trop Med Hyg. (1975) 78:194–200. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

