Differentially Expressed Genes in the Brain of Aging Mice With Cognitive Alteration and Depression- and Anxiety-Like Behaviors
- PMID: 33015035
- PMCID: PMC7493670
- DOI: 10.3389/fcell.2020.00814
Differentially Expressed Genes in the Brain of Aging Mice With Cognitive Alteration and Depression- and Anxiety-Like Behaviors
Abstract
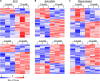
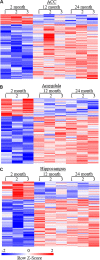
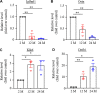
Despite the great increase in human lifespan with improved medical care, the physiological and pathological changes such as memory and cognitive disorders and associated anxiety and depression are major concern with aging. Molecular mechanisms underlying these changes are little known. The present study examined the differentially expressed genes (DEGs) and the genes with differentially expressed isoforms in three brain regions, anterior cingulate cortex (ACC), amygdala and hippocampus, throughout the lifespan of mice. Compared to 2-month old mice, both 12- and 24-month old mice displayed memory and cognitive impairments in the Morris water maze, Y-maze, and novel object recognition tests and depression- and anxiety-like behaviors in the tail suspension, forced swimming, open field, and elevated plus maze tests. RNA sequencing analysis identified 634 and 1078 DEGs in ACC, 453 and 1015 DEGs in the amygdala and 884 and 1054 DEGs in hippocampus in the 12- and 24-month old mice, respectively. Similarly, many genes with differentially expressed isoforms were also identified in these three brain regions in the 12- and 24-month old mice. Further functional analysis revealed that many DEGs and the genes with differentially expressed isoforms in the ACC and amygdala were mapped to depression- and anxiety-related genes, respectively and that a lot of DEGs and the genes with differentially expressed isoforms in hippocampus were mapped to cognitive dysfunction-related genes from both 12- and 24-month old mice. All of these mapped DEGs and the genes with differentially expressed isoforms were closely related to neuroinflammation. Our findings indicate that these neuroinflammation-related DEGs and the genes with differentially expressed isoforms are likely new targets in the management of memory/cognitive impairment and emotional disorders during the aging.
Keywords: RNA sequencing; aging mice; anxiety; cognitive dysfunction; depression.
Copyright © 2020 Li, Su, Cai, Cao, Miao, Zang, Gao, Xu, Yang, Tao and Ai.
Figures





References
-
- Bach M. E., Barad M., Son H., Zhuo M., Lu Y. F., Shih R., et al. (1999). Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 96 5280–5285. 10.1073/pnas.96.9.5280 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

