Caffeine Targets SIRT3 to Enhance SOD2 Activity in Mitochondria
- PMID: 33015038
- PMCID: PMC7493682
- DOI: 10.3389/fcell.2020.00822
Caffeine Targets SIRT3 to Enhance SOD2 Activity in Mitochondria
Abstract
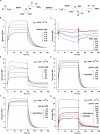
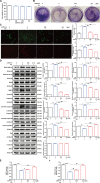
Caffeine is chemically stable and not readily oxidized under normal physiological conditions but also has antioxidant effects, although the underlying molecular mechanism is not well understood. Superoxide dismutase (SOD) 2 is a manganese-containing enzyme located in mitochondria that protects cells against oxidative stress by scavenging reactive oxygen species (ROS). SOD2 activity is inhibited through acetylation under conditions of stress such as exposure to ultraviolet (UV) radiation. Sirtuin 3 (SIRT3) is the major mitochondrial nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, which deacetylates two critical lysine residues (lysine 68 and lysine 122) on SOD2 and promotes its antioxidative activity. In this study, we investigated whether the antioxidant effect of caffeine involves modulation of SOD2 by SIRT3 using in vitro and in vivo models. The results show that caffeine interacts with SIRT3 and promotes direct binding of SIRT3 with its substrate, thereby enhancing its enzymatic activity. Mechanistically, caffeine bound to SIRT3 with high affinity (K D = 6.858 × 10-7 M); the binding affinity between SIRT3 and its substrate acetylated p53 was also 9.03 (without NAD+) or 6.87 (with NAD+) times higher in the presence of caffeine. Caffeine effectively protected skin cells from UV irradiation-induced oxidative stress. More importantly, caffeine enhanced SIRT3 activity and reduced SOD2 acetylation, thereby leading to increased SOD2 activity, which could be reversed by treatment with the SIRT3 inhibitor 3-(1H-1,2,3-triazol-4-yl) pyridine (3-TYP) in vitro and in vivo. Taken together, our results show that caffeine targets SIRT3 to enhance SOD2 activity and protect skin cells from UV irradiation-induced oxidative stress. Thus, caffeine, as a small-molecule SIRT3 activator, could be a potential agent to protect human skin against UV radiation.
Keywords: SIRT3; SOD2; UV radiation; antioxidant effects; caffeine; skin photoprotection.
Copyright © 2020 Xu, Gan, Gao, Huang, Wu, Zhang, Wang and Sheng.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

