PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance
- PMID: 33015058
- PMCID: PMC7509090
- DOI: 10.3389/fcell.2020.564601
PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance
Abstract
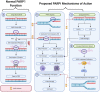
The Poly (ADP-ribose) polymerase (PARP) family has many essential functions in cellular processes, including the regulation of transcription, apoptosis and the DNA damage response. PARP1 possesses Poly (ADP-ribose) activity and when activated by DNA damage, adds branched PAR chains to facilitate the recruitment of other repair proteins to promote the repair of DNA single-strand breaks. PARP inhibitors (PARPi) were the first approved cancer drugs that specifically targeted the DNA damage response in BRCA1/2 mutated breast and ovarian cancers. Since then, there has been significant advances in our understanding of the mechanisms behind sensitization of tumors to PARP inhibitors and expansion of the use of PARPi to treat several other cancer types. Here, we review the recent advances in the proposed mechanisms of action of PARPi, biomarkers of the tumor response to PARPi, clinical advances in PARPi therapy, including the potential of combination therapies and mechanisms of tumor resistance.
Keywords: BRCA; DNA damage; DNA repair; PARP inhibitors; cancer; targeted therapy.
Copyright © 2020 Rose, Burgess, O’Byrne, Richard and Bolderson.
Figures
References
-
- Abida W., Campbell D., Patnaik A., Sautois B., Shapiro J., Vogelzang N. J., et al. (2019). 846PD - Preliminary results from the TRITON2 study of rucaparib in patients (pts) with DNA damage repair (DDR)-deficient metastatic castration-resistant prostate cancer (mCRPC): updated analyses. Ann. Oncol. 30 v327–v328. 10.1093/annonc/mdz248.003 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

