Variants of the human RAD52 gene confer defects in ionizing radiation resistance and homologous recombination repair in budding yeast
- PMID: 33015141
- PMCID: PMC7517009
- DOI: 10.15698/mic2020.10.732
Variants of the human RAD52 gene confer defects in ionizing radiation resistance and homologous recombination repair in budding yeast
Abstract
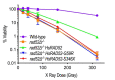
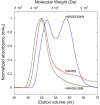
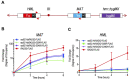
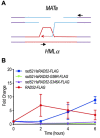
RAD52 is a structurally and functionally conserved component of the DNA double-strand break (DSB) repair apparatus from budding yeast to humans. We recently showed that expressing the human gene, HsRAD52 in rad52 mutant budding yeast cells can suppress both their ionizing radiation (IR) sensitivity and homologous recombination repair (HRR) defects. Intriguingly, we observed that HsRAD52 supports DSB repair by a mechanism of HRR that conserves genome structure and is independent of the canonical HR machinery. In this study we report that naturally occurring variants of HsRAD52, one of which suppresses the pathogenicity of BRCA2 mutations, were unable to suppress the IR sensitivity and HRR defects of rad52 mutant yeast cells, but fully suppressed a defect in DSB repair by single-strand annealing (SSA). This failure to suppress both IR sensitivity and the HRR defect correlated with an inability of HsRAD52 protein to associate with and drive an interaction between genomic sequences during DSB repair by HRR. These results suggest that HsRAD52 supports multiple, distinct DSB repair apparatuses in budding yeast cells and help further define its mechanism of action in HRR. They also imply that disruption of HsRAD52-dependent HRR in BRCA2-defective human cells may contribute to protection against tumorigenesis and provide a target for killing BRCA2-defective cancers.
Keywords: DNA double strand breaks; HsRAD52 variants; budding yeast; homologous recombination repair; ionizing radiation; tumorigenesis.
Copyright: © 2020 Clear et al.
Conflict of interest statement
Conflict of interest: The authors declare that there are no conflicts of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
