Resistant Maltodextrin Alleviates Dextran Sulfate Sodium-Induced Intestinal Inflammatory Injury by Increasing Butyric Acid to Inhibit Proinflammatory Cytokine Levels
- PMID: 33015180
- PMCID: PMC7519446
- DOI: 10.1155/2020/7694734
Resistant Maltodextrin Alleviates Dextran Sulfate Sodium-Induced Intestinal Inflammatory Injury by Increasing Butyric Acid to Inhibit Proinflammatory Cytokine Levels
Abstract
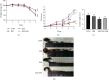
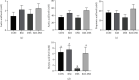
Inflammatory bowel disease (IBD), one kind of intestinal chronic inflammatory disease, is characterized by colonic epithelial barrier injury, overproduction of proinflammatory cytokines, and fewer short-chain fatty acids (SCFAs). The present study is aimed at testing the hypothesis that resistant maltodextrin (RM), a soluble dietary fiber produced by starch debranching, alleviated dextran sulfate sodium- (DSS-) induced colitis in mice. Female C57BL/6 mice with or without oral administration of 50 mg/kg RM for 19 days were challenged with 3% DSS in drinking water to induce colitis (from day 14 to day 19). Although RM could not reverse DSS-induced weight loss or colon shortening, it reduced inflammatory cell infiltration and epithelial damage in colon tissue, as well as the transfer of intestinal permeability indicators including serum diamine oxidase (DAO) and D-lactic acid (D-LA). ELISA analysis indicated that RM significantly suppressed the increase of Th1 cytokines induced by DSS in the colon such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ). The levels of proinflammatory cytokines interleukin-1β (IL-1β), IL-17, and IL-8 in the DSS group were significantly higher than those in the control group and RM group, but no significant difference was observed in the RM-DSS group compared with the RM group. Interestingly, IL-10 levels of the DSS group were significantly higher than those of the other groups. With respect to SCFAs, DSS administration significantly decreased the concentration of faecal butyric acid while the RM-DSS group showed a tendency to increase (P = 0.08). In general, RM alleviated dextran sulfate sodium-induced intestinal inflammation through increasing the level of butyric acid and subsequently inhibiting the expression of proinflammatory cytokines.
Copyright © 2020 Shilan Wang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



Similar articles
-
Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis.World J Gastroenterol. 2017 Feb 14;23(6):999-1009. doi: 10.3748/wjg.v23.i6.999. World J Gastroenterol. 2017. PMID: 28246473 Free PMC article.
-
Zhikang Capsule ameliorates dextran sodium sulfate-induced colitis by inhibition of inflammation, apoptosis, oxidative stress and MyD88-dependent TLR4 signaling pathway.J Ethnopharmacol. 2016 Nov 4;192:236-247. doi: 10.1016/j.jep.2016.07.055. Epub 2016 Jul 21. J Ethnopharmacol. 2016. PMID: 27452656
-
Sodium chloride exacerbates dextran sulfate sodium-induced colitis by tuning proinflammatory and antiinflammatory lamina propria mononuclear cells through p38/MAPK pathway in mice.World J Gastroenterol. 2018 Apr 28;24(16):1779-1794. doi: 10.3748/wjg.v24.i16.1779. World J Gastroenterol. 2018. PMID: 29713131 Free PMC article.
-
Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon.Am J Physiol Gastrointest Liver Physiol. 2017 Jun 1;312(6):G550-G558. doi: 10.1152/ajpgi.00256.2016. Epub 2017 Mar 30. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28360029
-
Does caffeine have a double-edged sword role in inflammation and carcinogenesis in the colon?Intest Res. 2023 Jul;21(3):306-317. doi: 10.5217/ir.2022.00118. Epub 2023 Apr 20. Intest Res. 2023. PMID: 37072923 Free PMC article. Review.
Cited by
-
Integrative analysis with microbial modelling and machine learning uncovers potential alleviators for ulcerative colitis.Gut Microbes. 2024 Jan-Dec;16(1):2336877. doi: 10.1080/19490976.2024.2336877. Epub 2024 Apr 2. Gut Microbes. 2024. PMID: 38563656 Free PMC article.
-
Tumorigenesis in Inflammatory Bowel Disease: Microbiota-Environment Interconnections.Cancers (Basel). 2023 Jun 15;15(12):3200. doi: 10.3390/cancers15123200. Cancers (Basel). 2023. PMID: 37370812 Free PMC article. Review.
-
Alginate Alleviates Dextran Sulfate Sodium-Induced Colitis by Promoting Bifidobacterium animalis and Intestinal Hyodeoxycholic Acid Synthesis in Mice.Microbiol Spectr. 2022 Dec 21;10(6):e0297922. doi: 10.1128/spectrum.02979-22. Epub 2022 Oct 11. Microbiol Spectr. 2022. PMID: 36219101 Free PMC article.
-
Validity of food additive maltodextrin as placebo and effects on human gut physiology: systematic review of placebo-controlled clinical trials.Eur J Nutr. 2022 Sep;61(6):2853-2871. doi: 10.1007/s00394-022-02802-5. Epub 2022 Mar 1. Eur J Nutr. 2022. PMID: 35230477 Free PMC article.
-
Industrial Bread Composition: Potential Implications for Patients with Inflammatory Bowel Disease.Nutrients. 2025 Jun 26;17(13):2120. doi: 10.3390/nu17132120. Nutrients. 2025. PMID: 40647225 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

