Antihypernociceptive, Anxiolytic, and Antidepressant Properties of Aqueous and Ethanol Extracts of Dissotis thollonii Cogn. (Melastomataceae) in Mice
- PMID: 33015629
- PMCID: PMC7525301
- DOI: 10.1155/2020/8886894
Antihypernociceptive, Anxiolytic, and Antidepressant Properties of Aqueous and Ethanol Extracts of Dissotis thollonii Cogn. (Melastomataceae) in Mice
Abstract
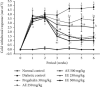
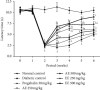
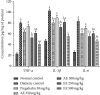
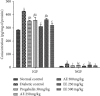
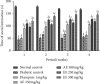
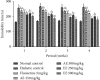
Diabetic neuropathy, which affects 7 to 9% of the world's population and that is usually accompanied by anxiety and depression, is chronic pain that results from impaired function of the central or peripheral nervous system. This study aimed at evaluating the antihypernociceptive, antiallodynic, anxiolytic, and antidepressant effects of Dissotis thollonii extracts. Diabetic neuropathy was induced by intraperitoneal injection of streptozotocin (200 mg/kg) in mice. The aqueous and ethanol extracts (250 and 500 mg/kg) were administered orally. Hyperalgesia (thermal and chemical), allodynia (mechanical and thermal), anxiety (high plus labyrinth, light-dark box, and social interaction), and depression (open field test, suspension test tail, and forced swimming test) were evaluated, and then the levels of some cytokines and growth factors were determined. The aqueous and ethanol extracts of Dissotis thollonii demonstrated significant antihypernociceptive (inhibition of hyperalgesia and allodynia), anxiolytic, and antidepressant activities in mice made diabetic by STZ. The extracts also significantly inhibited (p < 0.001) the levels of TNF-α, IL-1β, and IL-6 in the blood as well as the levels of TNF-α, IL-1β, IL-6, IGF, and NGF in the sciatic nerve. This study shows that the extracts of Dissotis thollonii have antihypernociceptive and neuroprotective effects which could be linked to the inhibition of proinflammatory cytokines and growth factors in the blood and the sciatic nerve.
Copyright © 2020 Stephanie Flore Djuichou Nguemnang et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures







References
-
- Mbiantcha M., Almas J., Shabana U. S., Nida D., Aisha F. Anti-arthritic property of crude extracts of piptadeniastrum africanum (Mimosaceae) in complete freund’s adjuvant-induced arthritis in rats. Complementary and Alternative Medicine. 2017;17(1):1–16. doi: 10.1186/s12906-017-1623-5. - DOI - PMC - PubMed
-
- Babaei-Balderlou F., Zare S., Heidari R., Farrokhi F. Effects of melatonin and vitamin E on peripheral neuropathic pain in streptozotocin-induced diabetic rats. Iranian Journal of Basic Medical Sciences. 2010;13(2):1–8.
LinkOut - more resources
Full Text Sources
