Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study
- PMID: 33015652
- PMCID: PMC7518831
- DOI: 10.1016/S2666-5247(20)30120-8
Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic. The proportion of infected individuals who seroconvert is still an open question. In addition, it has been shown in some individuals that viral genome can be detected up to 3 months after symptom resolution. We investigated both seroconversion and PCR positivity in a large cohort of convalescent serum donors in the New York City (NY, USA) region.
Methods: In this observational study, we ran an outreach programme in the New York City area. We recruited participants via the REDCap (Vanderbilt University, Nashville, TN, USA) online survey response. Individuals with confirmed or suspected SARS-CoV-2 infection were screened via PCR for presence of viral genome and via ELISA for presence of anti-SARS-CoV-2 spike antibodies. One-way ANOVA and Fisher's exact test were used to measure the association of age, gender, symptom duration, and days from symptom onset and resolution with positive antibody results.
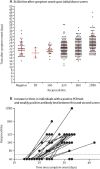
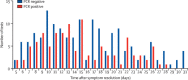
Findings: Between March 26 and April 10, 2020, we measured SARS-CoV-2 antibody titres in 1343 people. Of the 624 participants with confirmed SARS-CoV-2 infection who had serologies done after 4 weeks, all but three seroconverted to the SARS-CoV-2 spike protein, whereas 269 (37%) of 719 participants with suspected SARS-CoV-2 infection seroconverted. PCR positivity was detected up to 28 days from symptom resolution.
Interpretation: Most patients with confirmed COVID-19 seroconvert, potentially providing immunity to reinfection. We also report that in a large proportion of individuals, viral genome can be detected via PCR in the upper respiratory tract for weeks after symptom resolution, but it is unclear whether this signal represents infectious virus. Analysis of our large cohort suggests that most patients with mild COVID-19 seroconvert 4 weeks after illness, and raises questions about the use of PCR to clear positive individuals.
Funding: None.
© 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Figures
References
-
- Chu CM, Leung WS, Cheng VC. Duration of RT-PCR positivity in severe acute respiratory syndrome. Eur Respir J. 2005;25:12–14. - PubMed
-
- Wu F, Wang A, Liu M. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 doi: 10.1101/2020.03.30.20047365. published online April 20. (preprint) - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

