Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study
- PMID: 33015653
- PMCID: PMC7518879
- DOI: 10.1016/S2666-5247(20)30144-0
Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) targets multiple organs and causes severe coagulopathy. Histopathological organ changes might not only be attributable to a direct virus-induced effect, but also the immune response. The aims of this study were to assess the duration of viral presence, identify the extent of inflammatory response, and investigate the underlying cause of coagulopathy.
Methods: This prospective autopsy cohort study was done at Amsterdam University Medical Centers (UMC), the Netherlands. With informed consent from relatives, full body autopsy was done on 21 patients with COVID-19 for whom autopsy was requested between March 9 and May 18, 2020. In addition to histopathological evaluation of organ damage, the presence of SARS-CoV-2 nucleocapsid protein and the composition of the immune infiltrate and thrombi were assessed, and all were linked to disease course.
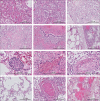
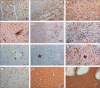
Findings: Our cohort (n=21) included 16 (76%) men, and median age was 68 years (range 41-78). Median disease course (time from onset of symptoms to death) was 22 days (range 5-44 days). In 11 patients tested for SARS-CoV-2 tropism, SARS-CoV-2 infected cells were present in multiple organs, most abundantly in the lungs, but presence in the lungs became sporadic with increased disease course. Other SARS-CoV-2-positive organs included the upper respiratory tract, heart, kidneys, and gastrointestinal tract. In histological analyses of organs (sampled from nine to 21 patients per organ), an extensive inflammatory response was present in the lungs, heart, liver, kidneys, and brain. In the brain, extensive inflammation was seen in the olfactory bulbs and medulla oblongata. Thrombi and neutrophilic plugs were present in the lungs, heart, kidneys, liver, spleen, and brain and were most frequently observed late in the disease course (15 patients with thrombi, median disease course 22 days [5-44]; ten patients with neutrophilic plugs, 21 days [5-44]). Neutrophilic plugs were observed in two forms: solely composed of neutrophils with neutrophil extracellular traps (NETs), or as aggregates of NETs and platelets..
Interpretation: In patients with lethal COVID-19, an extensive systemic inflammatory response was present, with a continued presence of neutrophils and NETs. However, SARS-CoV-2-infected cells were only sporadically present at late stages of COVID-19. This suggests a maladaptive immune response and substantiates the evidence for immunomodulation as a target in the treatment of severe COVID-19.
Funding: Amsterdam UMC Corona Research Fund.
© 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Figures


Comment in
-
Immunogenomic phases of COVID-19 and appropriate clinical management.Lancet Microbe. 2020 Nov;1(7):e278. doi: 10.1016/S2666-5247(20)30165-8. Epub 2020 Nov 4. Lancet Microbe. 2020. PMID: 33521726 Free PMC article. No abstract available.
-
Neutrophils as a pallbearer for SARS-CoV-2 disease burden.Lancet Microbe. 2021 Feb;2(2):e56. doi: 10.1016/S2666-5247(21)00002-1. Epub 2021 Feb 2. Lancet Microbe. 2021. PMID: 33655231 Free PMC article. No abstract available.
-
Neutrophils as a pallbearer for SARS-CoV-2 disease burden - Authors' reply.Lancet Microbe. 2021 Feb;2(2):e57. doi: 10.1016/S2666-5247(21)00001-X. Epub 2021 Feb 2. Lancet Microbe. 2021. PMID: 33655232 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

