Predicting susceptibility to SARS-CoV-2 infection based on structural differences in ACE2 across species
- PMID: 33015868
- PMCID: PMC7675292
- DOI: 10.1096/fj.202001808R
Predicting susceptibility to SARS-CoV-2 infection based on structural differences in ACE2 across species
Abstract
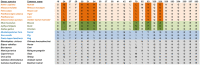
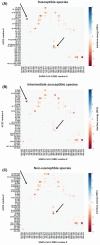
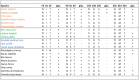
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the global pandemic of coronavirus disease-2019 (COVID-19). SARS-CoV-2 is a zoonotic disease, but little is known about variations in species susceptibility that could identify potential reservoir species, animal models, and the risk to pets, wildlife, and livestock. Certain species, such as domestic cats and tigers, are susceptible to SARS-CoV-2 infection, while other species such as mice and chickens are not. Most animal species, including those in close contact with humans, have unknown susceptibility. Hence, methods to predict the infection risk of animal species are urgently needed. SARS-CoV-2 spike protein binding to angiotensin-converting enzyme 2 (ACE2) is critical for viral cell entry and infection. Here we integrate species differences in susceptibility with multiple in-depth structural analyses to identify key ACE2 amino acid positions including 30, 83, 90, 322, and 354 that distinguish susceptible from resistant species. Using differences in these residues across species, we developed a susceptibility score that predicts an elevated risk of SARS-CoV-2 infection for multiple species including horses and camels. We also demonstrate that SARS-CoV-2 is nearly optimal for binding ACE2 of humans compared to other animals, which may underlie the highly contagious transmissibility of this virus among humans. Taken together, our findings define potential ACE2 and SARS-CoV-2 residues for therapeutic targeting and identification of animal species on which to focus research and protection measures for environmental and public health.
Keywords: COVID-19; angiotensin-converting enzyme 2; protein structural elements; severe acute respiratory syndrome coronavirus 2.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare that there are no conflicts of interest in connection with this article.
Figures






References
-
- APHISpress@usda.gov . (April 6, 2020). USDA Statement on the Confirmation of COVID‐19 in a Tiger in New York. United States Department of Agriculture Animal and Plant Health Inspection Service. https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa‐2020/ny‐zoo...
-
- APHISpress@usda.gov . (August 13, 2020). Confirmed cases of SARS‐CoV‐2 in animals in the United States. United States Department of Agriculture Animal and Plant Health Inspection Service. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/SA_One_Health/sar...
Publication types
MeSH terms
Substances
Grants and funding
- F30 HL151069/HL/NHLBI NIH HHS/United States
- R01AI141661/NH/NIH HHS/United States
- EIA34480023/American Heart Association/International
- U01 AI150739/AI/NIAID NIH HHS/United States
- DP2 HL137166/HL/NHLBI NIH HHS/United States
- U19 AI117905/AI/NIAID NIH HHS/United States
- DK117147/NH/NIH HHS/United States
- R35 GM127087/GM/NIGMS NIH HHS/United States
- R01 DK117147/DK/NIDDK NIH HHS/United States
- R35GM127087/NH/NIH HHS/United States
- F32 HL144048/HL/NHLBI NIH HHS/United States
- F32HL144048-01/NH/NIH HHS/United States
- U01AI150739/NH/NIH HHS/United States
- R01 AI141661/AI/NIAID NIH HHS/United States
- U01TR002383/NH/NIH HHS/United States
- U01 TR002383/TR/NCATS NIH HHS/United States
- UH3TR002097/NH/NIH HHS/United States
- DP2HL137166/NH/NIH HHS/United States
- K08 HL153786/HL/NHLBI NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- 20PRE35080177/American Heart Association/International
- T32 GM152284/GM/NIGMS NIH HHS/United States
- U19AI117905/NH/NIH HHS/United States
- UH3 TR002097/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

