Disruption in the balance between apolipoprotein A-I and mast cell chymase in chronic hypersensitivity pneumonitis
- PMID: 33016012
- PMCID: PMC7654418
- DOI: 10.1002/iid3.355
Disruption in the balance between apolipoprotein A-I and mast cell chymase in chronic hypersensitivity pneumonitis
Abstract
Background: Apolipoprotein A-I (apoA-I) has an antifibrotic effect in idiopathic pulmonary fibrosis. Although pulmonary fibrosis is associated with poor prognosis of patients with hypersensitivity pneumonitis (HP), little is known regarding the role of apoA-I in the pathogenesis of HP.
Methods: Two-dimensional electrophoresis, immunoblotting, and enzyme-linked immunosorbent assays were performed for the identification and quantification of apoA-I in bronchoalveolar lavage fluid (BALF) from patients with acute and chronic HP. To investigate the degradation of apoA-I, apoA-I was incubated with BALF. Moreover, the role of apoA-I in TGF-β1-induced epithelial-mesenchymal transition of A549 cells was examined.
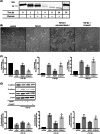
Results: The concentration of apoA-I in the BALF was significantly lower in chronic HP (n = 56) compared with acute HP (n = 31). The expression level of apoA-I was also low in the lung tissues of chronic HP. ApoA-I was degraded by BALF from HP patients. The number of chymase-positive mast cells in the alveolar parenchyma was inversely correlated with apoA-I levels in the BALF of chronic HP patients. In vitro experiment using A549 cells, untreated apoA-I inhibited TGF-β1-induced epithelial-mesenchymal transition, although this trend was not observed in the chymase-treated apoA-I.
Conclusions: A decrease of apoA-I was associated with the pathogenesis of chronic HP in terms of pulmonary fibrosis and mast cell chymase attenuated the protective effect of apoA-I against pulmonary fibrosis. Furthermore, apoA-I could be a crucial molecule associated with lung fibrogenesis of HP.
Keywords: apolipoprotein A-I; chymase; hypersensitivity pneumonitis; mast cell.
© 2020 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures





References
-
- Fink JN. Hypersensitivity pneumonitis. J Allergy Clin Immunol. 1984;74:1‐10. - PubMed
-
- Inoue Y, Ishizuka M, Furusawa H, et al. Acute inflammatory and immunologic responses against antigen in chronic bird‐related hypersensitivity pneumonitis. Allergol Int. 2019;68:321‐328. - PubMed
-
- Lacasse Y, Selman M, Costabel U, et al. Classification of hypersensitivity pneumonitis: a hypothesis. Int Arch Allergy Immunol. 2009;149:161‐166. - PubMed
-
- Vourlekis JS, Schwarz MI, Cherniack RM, et al. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004;116:662‐668. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

