Streamlined Catalytic Enantioselective Synthesis of α-Substituted β,γ-Unsaturated Ketones and Either of the Corresponding Tertiary Homoallylic Alcohol Diastereomers
- PMID: 33016068
- PMCID: PMC7775104
- DOI: 10.1021/jacs.0c08732
Streamlined Catalytic Enantioselective Synthesis of α-Substituted β,γ-Unsaturated Ketones and Either of the Corresponding Tertiary Homoallylic Alcohol Diastereomers
Abstract
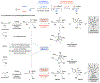
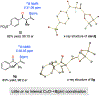
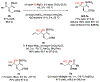
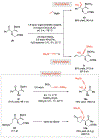
A widely applicable, practical, and scalable strategy for efficient and enantioselective synthesis of β,γ-unsaturated ketones that contain an α-stereogenic center is disclosed. Accordingly, aryl, heteroaryl, alkynyl, alkenyl, allyl, or alkyl ketones that contain an α-stereogenic carbon with an alkyl, an aryl, a benzyloxy, or a siloxy moiety can be generated from readily available starting materials and by the use of commercially available chiral ligands in 52-96% yield and 93:7 to >99:1 enantiomeric ratio. To develop the new method, conditions were identified so that high enantioselectivity would be attained and the resulting α-substituted NH-ketimines, wherein there is strong C═N → B(pin) coordination, would not epimerize before conversion to the derived ketone by hydrolysis. It is demonstrated that the ketone products can be converted to an assortment of homoallylic tertiary alcohols in 70-96% yield and 92:8 to >98:2 dr-in either diastereomeric form-by reactions with alkyl-, aryl-, heteroaryl-, allyl-, vinyl-, alkynyl-, or propargyl-metal reagents. The utility of the approach is highlighted through transformations that furnish other desirable derivatives and a concise synthesis route affording more than a gram of a major fragment of anti-HIV agents rubriflordilactones A and B and a specific stereoisomeric analogue.
Figures

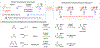








References
-
- Ravindranath N; Venkataiah B; Ramesh C; Jayaprakash P; Das B Jatrophenone, a novel macrocyclic bioactive diterpene from Jatropha gossypifolia. Chem. Pharm. Bull. 2003, 51, 870–871. - PubMed
-
- Pimentel-Elardo SM; Gulder TAM; Hentschel U; Bringmann G Cebulactams A1 and A2, new macrolactams isolated from Saccaropolyspora cebuensis, the first obligate marine strain of the genus Saccharopolyspora. Tetrahedron Lett. 2008, 49, 6889–6892.
- Xie C-L; Niu S; Xia J-M; Peng K; Zhang G-Y; Yang X-W Saccharopolytide A, a new cyclic tetrapeptide with rare 4-hydroxy-proline moieties from the deep-sea derived actinomycete Saccharopolyspora cebuensis MCCC 1A09850. Nat. Prod. Res. 2018, 32, 1627–1631. - PubMed
-
- Yan X-X; Liang C-G; Zhang Y; Hong W; Cao B-X; Dai L-X; Hou X-L Highly enantioselective Pd-catalyzed allylic alkylations of acyclic ketones. Angew. Chem., Int. Ed. 2005, 44, 6544–6546. - PubMed
- Trost BM; Xu J Palladium-catalyzed asymmetric allylic α-alkylation of acyclic ketones. J. Am. Chem. Soc. 2005, 127, 17180–17181. - PMC - PubMed
- Zheng W-H; Zheng B-H; Zhang Y; Hou X-L Highly regio-, diastereo-, and enantioselective Pd-catalyzed allylic alkylation of acyclic ketone enolates with monosubstituted allyl substrates. J. Am. Chem. Soc. 2007, 129, 7718–7719. - PubMed
- Lundin PM; Esquivias J; Fu GC Catalytic asymmetric cross-couplings of racemic α-bromoketones with arylzinc reagents. Angew. Chem., Int. Ed. 2009, 48, 154–156. - PMC - PubMed
- Lou S; Fu GC Nickel/bis(oxazoline)-catalyzed asymmetric Kumada reactions of alkyl electrophiles: cross-couplings of racemic α-bromoketones. J. Am. Chem. Soc. 2010, 132, 1264–1266. - PMC - PubMed
- Cherney AH; Kadunce NT; Reisman SE Catalytic asymmetric reductive acyl cross-coupling: synthesis of enantioenriched acyclic α,α-disubstituted ketones. J. Am. Chem. Soc. 2013, 135, 7442–7445. - PubMed
- Bandar JS; Ascic E; Buchwald SL Enantioselective CuH-catalyzed reductive coupling of aryl alkenes and activated carboxylic acids. J. Am. Chem. Soc. 2016, 138, 5821–5824. - PMC - PubMed
- Zhou Y; Bandar JS; Buchwald SL Enantioselective CuH- catalyzed hydroacylation employing unsaturated carboxylic acids as aldehyde surrogates. J. Am. Chem. Soc. 2017, 139, 8126–8129. - PMC - PubMed
- Liu J; Vasamsetty L; Anwar M; Yang S; Xu W; Liu J; Nagaraju S; Fang X Organocatalyzed kinetic resolution of α-functionalized ketones: the malonate unit leads the way. ACS Catal. 2020, 10, 2882–2893.
-
- Chen J-P; Ding C-H; Liu W; Hou X-L; Dai L-X Palladium-catalyzed regio-, diastereo-, and enantioselective alkylation of acylsilanes with monosubstituted allyl substrates. J. Am. Chem. Soc. 2010, 132, 15493–15495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

