Racial and Ethnic Differences in Biomarkers, Health Status, and Cardiac Remodeling in Patients With Heart Failure With Reduced Ejection Fraction Treated With Sacubitril/Valsartan
- PMID: 33016100
- PMCID: PMC7769180
- DOI: 10.1161/CIRCHEARTFAILURE.120.007829
Racial and Ethnic Differences in Biomarkers, Health Status, and Cardiac Remodeling in Patients With Heart Failure With Reduced Ejection Fraction Treated With Sacubitril/Valsartan
Abstract
Background: Among patients with heart failure and reduced ejection fraction (left ventricular (LV) ejection fraction ≤40%), sacubitril/valsartan (S/V) treatment is associated with improved health status and reverse cardiac remodeling. Data regarding racial and ethnic differences in response to S/V are lacking.
Methods: This was an analysis from the PROVE-HF study (Prospective Study of Biomarkers, Symptom Improvement and Ventricular Remodeling During Entresto Therapy for Heart Failure). Longitudinal changes in NT-proBNP (N-terminal pro-B-type natriuretic peptide), cardiac reverse remodeling, and health status scores were compared between groups using multivariate latent growth curve modeling.
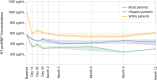
Results: Among the 782 patients included in this study, 22.7% were non-Hispanic Black (from here referred to as Black), 14.9% were Hispanic, and 62.4% were non-Hispanic White (from here referred to as White). At baseline, compared with White patients, Black and Hispanic patients had lower NT-proBNP (g=0.34) and differences between groups in baseline values for LV end-diastolic volume index and LV end-systolic volume index were negligible (g<0.10). Following S/V initiation, NT-proBNP decreased in all 3 groups (P<0.0001) associated with improvements in LV ejection fraction, LV end-diastolic volume index, and LV end-systolic volume index. Although total improvement in LV measures was similar between groups, Black patients averaged larger gains in the first half of the trial while White patients averaged larger gains in the second half. Improvements in Kansas City Cardiomyopathy Questionnaire-23 Total Symptom scores were seen in all 3 groups. Treatment with S/V was well-tolerated.
Conclusions: Among Black, Hispanic, and White patients with heart failure and reduced ejection fraction, treatment with S/V was associated with similar reduction in NT-proBNP, improvement in health status, and reverse remodeling. More data regarding racial and ethnic responses to heart failure and reduced ejection fraction treatment are needed. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02887183.
Keywords: biomarker; health status; heart failure; ventricular remodeling.
Conflict of interest statement
Dr Ibrahim has received speaker honoraria from Novartis and Roche Diagnostics. Dr Felker has received research grants from National Heart, Lung, and Blood Institute (NHLBI), American Heart Association, Amgen, Merck, Cytokinetics, and Roche Diagnostics; he has acted as a consultant to Novartis, Amgen, Bristol Myers Squibb (BMS), Cytokinetics, Medtronic, Cardionomic, Relypsa, V-Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Sphingotec, Roche Diagnostics, Alnylam, LivaNova, Rocket Pharma, and SC Pharma. Dr Maisel has received consulting income from Abbott Vascular, Ortho Clinical Diagnostics, and Novartis. Dr Butler is a consultant for Abbott, Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Relypsa, Vifor. Dr Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline (GSK), Ionis, Lone Star Heart, Mesoblast, MyoKardia, National Institutes of Health (NIH)/NHLBI, Novartis, Sanofi Pasteur, Theracos, and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya. Dr Prescott and C.A. Abbas are employees of Novartis Pharmaceuticals. Dr Januzzi is a Trustee of the American College of Cardiology, has received grant support from Novartis Pharmaceuticals and Abbott Diagnostics, consulting income from Abbott, Janssen, Novartis, and Roche Diagnostics, and participates in clinical end point committees/data safety monitoring boards for Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia and Takeda. The other authors report no conflicts.
Figures

References
-
- Sharma A, Colvin-Adams M, Yancy CW. Heart failure in African Americans: disparities can be overcome. Cleve Clin J Med. 2014;81:301–311. doi: 10.3949/ccjm.81a.13045 - PubMed
-
- Khariton Y, Nassif ME, Thomas L, Fonarow GC, Mi X, DeVore AD, Duffy C, Sharma PP, Albert NM, Patterson JH, et al. Health status disparities by sex, race/ethnicity, and socioeconomic status in outpatients with heart failure. JACC Heart Fail. 2018;6:465–473. doi: 10.1016/j.jchf.2018.02.002 - PMC - PubMed
-
- Bajaj NS, Gutiérrez OM, Arora G, Judd SE, Patel N, Bennett A, Prabhu SD, Howard G, Howard VJ, Cushman M, Arora P. Racial differences in plasma levels of N-terminal pro–B-type natriuretic peptide and outcomes: the reasons for geographic and racial differences in stroke (REGARDS) study. JAMA Cardiol. 2018;3:11–17. doi: 10.1001/jamacardio.2017.4207 - PMC - PubMed
-
- Benjamin Emelia J, Muntner P, Alonso A, Bittencourt Marcio S, Callaway Clifton W, Carson April P, Chamberlain Alanna M, Chang Alexander R, Cheng S, Das Sandeep R, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

