Placental microRNA expression associates with birthweight through control of adipokines: results from two independent cohorts
- PMID: 33016211
- PMCID: PMC8216179
- DOI: 10.1080/15592294.2020.1827704
Placental microRNA expression associates with birthweight through control of adipokines: results from two independent cohorts
Abstract
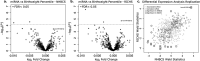
MicroRNAs are non-coding RNAs that regulate gene expression post-transcriptionally. In the placenta, the master regulator of foetal growth and development, microRNAs shape the basic processes of trophoblast biology and specific microRNA have been associated with foetal growth. To comprehensively assess the role of microRNAs in placental function and foetal development, we have performed small RNA sequencing to profile placental microRNAs from two independent mother-infant cohorts: the Rhode Island Child Health Study (n = 225) and the New Hampshire Birth Cohort Study (n = 317). We modelled microRNA counts on infant birthweight percentile (BWP) in each cohort, while accounting for race, sex, parity, and technical factors, using negative binomial generalized linear models. We identified microRNAs that were differentially expressed (DEmiRs) with BWP at false discovery rate (FDR) less than 0.05 in both cohorts. hsa-miR-532-5p (miR-532) was positively associated with BWP in both cohorts. By integrating parallel whole transcriptome and small RNA sequencing in the RICHS cohort, we identified putative targets of miR-532. These targets are enriched for pathways involved in adipogenesis, adipocytokine signalling, energy metabolism, and hypoxia response, and included Leptin, which we further demonstrated to have a decreasing expression with increasing BWP, particularly in male infants. Overall, we have shown a robust and reproducible association of miR-532 with BWP, which could influence BWP through regulation of adipocytokines Leptin and Adiponectin.
Keywords: adipokines; adiponectin; birthweight; hsa-miR-532-5p; leptin; microRNA; placenta; placental microRNA.
Conflict of interest statement
The authors have declared no competing interest.
Figures




Similar articles
-
Selenium-associated differentially expressed microRNAs and their targeted mRNAs across the placental genome in two U.S. birth cohorts.Epigenetics. 2022 Oct;17(10):1234-1245. doi: 10.1080/15592294.2021.2003044. Epub 2021 Nov 16. Epigenetics. 2022. PMID: 34784848 Free PMC article.
-
Placental microRNAs relate to early childhood growth trajectories.Pediatr Res. 2023 Jul;94(1):341-348. doi: 10.1038/s41390-022-02386-0. Epub 2022 Nov 15. Pediatr Res. 2023. PMID: 36380070 Free PMC article.
-
Copper associates with differential methylation in placentae from two US birth cohorts.Epigenetics. 2020 Mar;15(3):215-230. doi: 10.1080/15592294.2019.1661211. Epub 2019 Sep 4. Epigenetics. 2020. PMID: 31462129 Free PMC article.
-
Human placental microRNAs and preeclampsia.Biol Reprod. 2013 May 23;88(5):130. doi: 10.1095/biolreprod.113.107805. Print 2013 May. Biol Reprod. 2013. PMID: 23575145 Free PMC article. Review.
-
Adipokines, an adipose tissue and placental product with biological functions during pregnancy.Biofactors. 2012 Jan-Feb;38(1):14-23. doi: 10.1002/biof.201. Epub 2012 Jan 30. Biofactors. 2012. PMID: 22287297 Review.
Cited by
-
microRNAs in newborns with low birth weight: relation to birth size and body composition.Pediatr Res. 2022 Sep;92(3):829-837. doi: 10.1038/s41390-021-01845-4. Epub 2021 Nov 19. Pediatr Res. 2022. PMID: 34799665
-
Non-coding RNAs: the architects of placental development and pregnancy success.Mol Genet Genomics. 2025 Mar 30;300(1):39. doi: 10.1007/s00438-025-02244-8. Mol Genet Genomics. 2025. PMID: 40159439 Review.
-
Fetal Growth and Intrauterine Epigenetic Programming of Obesity and Cardiometabolic Disease.Neoreviews. 2022 Jun 1;23(6):e363-e372. doi: 10.1542/neo.23-6-e363. Neoreviews. 2022. PMID: 35641462 Free PMC article. Review.
-
A multi-omic approach identifies an autism spectrum disorder (ASD) regulatory complex of functional epimutations in placentas from children born preterm.Autism Res. 2023 May;16(5):918-934. doi: 10.1002/aur.2915. Epub 2023 Mar 20. Autism Res. 2023. PMID: 36938998 Free PMC article.
-
Coordinated Expressional Landscape of the Human Placental miRNome and Transcriptome.Front Cell Dev Biol. 2021 Jul 21;9:697947. doi: 10.3389/fcell.2021.697947. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34368147 Free PMC article.
References
-
- Quévillon Huberdeau M, Simard MJ. A guide to microRNA-mediated gene silencing. Febs J. 2019;286:642–652. - PubMed
-
- Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–282. - PubMed
-
- Hayder H, O’Brien J, Nadeem U, et al. MicroRNAs: crucial regulators of placental development. Reproduction. 2018;155:R259–R271. - PubMed
-
- Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
